Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Minari, Eriko*; Kabasawa, Satsuki; Mihara, Morihiro; Makino, Hitoshi; Asano, Hidekazu*; Nakase, Masahiko*; Takeshita, Kenji*
Journal of Nuclear Science and Technology, 60(7), p.793 - 803, 2023/07
Times Cited Count:2 Percentile:53.91(Nuclear Science & Technology)Okamura, Tomohiro*; Katano, Ryota; Oizumi, Akito; Nishihara, Kenji; Nakase, Masahiko*; Asano, Hidekazu*; Takeshita, Kenji*
Journal of Nuclear Science and Technology, 60(6), p.632 - 641, 2023/06
Times Cited Count:2 Percentile:53.91(Nuclear Science & Technology)The Okamura explicit method (OEM) for depletion calculation was developed by modifying the matrix exponential method for dynamic nuclear fuel cycle simulation. The OEM suppressed the divergence of the calculation for short half-life nuclides, even for long time steps. The computational cost of the OEM was small, equivalent to the Euler method, and it maintained sufficient accuracy for the fuel cycle simulation.
Sasaki, Yuji; Nakase, Masahiko*; Kaneko, Masashi; Kobayashi, Toru; Takeshita, Kenji*; Matsumiya, Masahiko*
Analytical Sciences, 5 Pages, 2023/00
Times Cited Count:0 Percentile:0(Chemistry, Analytical)We conducted three field researches on Ru-extraction, XANES, and DFT-calculation. The order of the distribution ratio, D(Ru), from acid, HCl  H
H SO
SO
 HNO
HNO
 HClO
HClO , by MIDOA is studied by XANES spectra, which indicates the valency change of Ru in HCl media and supports the ion pairing extraction of anionic Ru ion and cationic MIDOA. The same extractant trend, NTAamide
, by MIDOA is studied by XANES spectra, which indicates the valency change of Ru in HCl media and supports the ion pairing extraction of anionic Ru ion and cationic MIDOA. The same extractant trend, NTAamide  MIDOA
MIDOA  IDOA, due to D values as the energy gap of HOMO and LUMO could be found by DFT calculation, which suggests that the reaction heat has a positive correlation with extractability for extractant.
IDOA, due to D values as the energy gap of HOMO and LUMO could be found by DFT calculation, which suggests that the reaction heat has a positive correlation with extractability for extractant.
Okamura, Tomohiro*; Nishihara, Kenji; Katano, Ryota; Oizumi, Akito; Nakase, Masahiko*; Asano, Hidekazu*; Takeshita, Kenji*
JAEA-Data/Code 2021-016, 43 Pages, 2022/03
The quantitative prediction and analysis of the future nuclear energy utilization scenarios are required in order to establish the advanced nuclear fuel cycle. However, the nuclear fuel cycle consists of various processes from front- to back-end, and it is difficult to analyze the scenarios due to the complexity of modeling and the variety of scenarios. Japan Atomic Energy Agency and Tokyo Institute of Technology have jointly developed the NMB code as a tool for integrated analysis of mass balance from natural uranium needs to radionuclide migration of geological disposal. This user manual describes how to create a database and scenario input for the NMB version 4.0.
Okamura, Tomohiro*; Katano, Ryota; Oizumi, Akito; Nishihara, Kenji; Nakase, Masahiko*; Asano, Hidekazu*; Takeshita, Kenji*
Bulletin of the Laboratory for Advanced Nuclear Energy, 6, p.29 - 30, 2022/02
Takeshita Laboratory, Tokyo Institute of Technology, has been developing Nuclear Material Balance code version 4.0 (NMB4.0) in collaboration with Japan Atomic Energy Agency (JAEA). This report summarized the outline and functions of NMB4.0.
Sasaki, Yuji; Kaneko, Masashi; Ban, Yasutoshi; Matsumiya, Masahiko*; Nakase, Masahiko*; Takeshita, Kenji*
Separation Science and Technology, 57(16), p.2543 - 2553, 2022/00
Times Cited Count:3 Percentile:29.84(Chemistry, Multidisciplinary)The mutual separation of actinides (An) from lanthanides (Ln) using the masking agent of DTPA (diethylenetriamine-pentaacetic acid) or DTBA (diethylenetriamine-triacetic acid-bis(diethylacetamide)) in the aqueous phase through DGA extraction, referring TALSPEAK method, is focused. We investigate to obtain the same separation performance using commercially available DTPA on that using DTBA. In this work, we select lactic acid (LA) of pH buffer from 10 organic acids and ethylenediamine (ED) for the pH adjustment. Almost the same D and SF values are obtained among the conditions: TODGA-DTPA-LA-NaOH, TODGA-DTPA-LA-ED, and TODGA-DTBA-LA. The experimental results using batchwise multi-stage extractions show the average yields of Ln (La to Gd) and Am to be 3.73 and 98.1% in the aqueous phase using DGA-DTPA-LA-ED, to be 3.1 and 97.0% using DGA-DTPA-LA-NaOH, and to be 1.61 and 98.7% using DGA-DTBA-LA.
Sasaki, Yuji; Kaneko, Masashi; Matsumiya, Masahiko*; Nakase, Masahiko*; Takeshita, Kenji*
Solvent Extraction and Ion Exchange, 40(6), p.620 - 640, 2022/00
Times Cited Count:1 Percentile:14.86(Chemistry, Multidisciplinary)Owing to the chemical behavior of trivalent lanthanide and actinide ions with similar ionic radii, realizing this separation is still challenging. All lanthanides, Am, and Cm can be extracted using diglycolamide (DGA), and relatively high An/Ln separation efficiencies have been obtained using diethylenetriamine-triacetic-bisamide (DTBA). To improve the previous results as well as the separation conditions, we used organic acids for pH adjustment. The advantages of this modification included low HNO , DTBA concentrations and pH stability owing to the addition of lactic acid. Under these modified conditions, the recovery rates observed were as follows: 97.1% for Nd with the co-existence of 1.59% Am in organic phase, and 98.4% for Am with the co-existence of 2.95% Nd in aqueous phase.
, DTBA concentrations and pH stability owing to the addition of lactic acid. Under these modified conditions, the recovery rates observed were as follows: 97.1% for Nd with the co-existence of 1.59% Am in organic phase, and 98.4% for Am with the co-existence of 2.95% Nd in aqueous phase.
Okamura, Tomohiro*; Katano, Ryota; Oizumi, Akito; Nishihara, Kenji; Nakase, Masahiko*; Asano, Hidekazu*; Takeshita, Kenji*
EPJ Nuclear Sciences & Technologies (Internet), 7, p.19_1 - 19_13, 2021/11
Nuclear Material Balance code version 4.0 (NMB4.0) has been developed through collaborative R&D between Tokyo Institute of Technology and JAEA. Conventional nuclear fuel cycle simulation codes mainly analyze actinides and are specialized for front-end mass balance analysis. However, quantitative back-end simulation has recently become necessary for considering R&D strategies and sustainable nuclear energy utilization. Therefore, NMB4.0 was developed to realize the integrated nuclear fuel cycle simulation from front- to back-end. There are three technical features in NMB4.0: 179 nuclides are tracked, more than any other code, throughout the nuclear fuel cycle; the Okamura explicit method is implemented, which contributes to reducing the numerical cost while maintaining the accuracy of depletion calculations on nuclides with a shorter half-life; and flexibility of back-end simulation is achieved. The main objective of this paper is to show the newly developed functions, made for integrated back-end simulation, and verify NMB4.0 through a benchmark study to show the computational performance.
Kaneko, Masashi; Sasaki, Yuji; Wada, Eriko*; Nakase, Masahiko*; Takeshita, Kenji*
Chemistry Letters, 50(10), p.1765 - 1769, 2021/10
Times Cited Count:0 Percentile:0(Chemistry, Multidisciplinary)Density functional theory calculation is applied to predict the stability constants for Eu and Am
and Am complexes in aqueous solution for molecular modelling of novel separation agents for minor actinides over lanthanides. Logarithm of experimental stability constants correlates with calculated complex formation enthalpies with high reproducibility (R
complexes in aqueous solution for molecular modelling of novel separation agents for minor actinides over lanthanides. Logarithm of experimental stability constants correlates with calculated complex formation enthalpies with high reproducibility (R
 0.98). Prediction of stability constants of novel chelates is demonstrated and indicates a potential availability of the derivatives of diethylenetriaminepentaacetic acid type chelate in acidic condition and enhancement of Am
0.98). Prediction of stability constants of novel chelates is demonstrated and indicates a potential availability of the derivatives of diethylenetriaminepentaacetic acid type chelate in acidic condition and enhancement of Am selectivity over Eu
selectivity over Eu .
.
 /Eu
/Eu selectivity with diethylenetriaminepentaacetic acid and its bisamide chelates.
selectivity with diethylenetriaminepentaacetic acid and its bisamide chelates.Kaneko, Masashi; Sasaki, Yuji; Matsumiya, Masahiko*; Nakase, Masahiko*; Takeshita, Kenji*
Journal of Nuclear Science and Technology, 58(5), p.515 - 526, 2021/05
Times Cited Count:3 Percentile:35.51(Nuclear Science & Technology)Density-functional theory calculations were applied to molecular structure and complex formation reaction modelings of metal ion complexes with diethylenetriaminepentaacetic acid (DTPA) and its bisamide (DTPABA) chelates to understand the metal ions selectivity between Am and Eu
and Eu . The calculated complexes with DTPA and DTPABA chelates reproduced the coordination geometries of experimental crystal structures. Calculated Gibbs free energies of the complex formation reactions indicated that Am
. The calculated complexes with DTPA and DTPABA chelates reproduced the coordination geometries of experimental crystal structures. Calculated Gibbs free energies of the complex formation reactions indicated that Am ion forms higher stable complexes with both chelates than Eu
ion forms higher stable complexes with both chelates than Eu ion, being consistent with the experimental results. The higher Am
ion, being consistent with the experimental results. The higher Am selectivity over Eu
selectivity over Eu was suggested to originate in the larger bond overlap between Am
was suggested to originate in the larger bond overlap between Am 5f-orbital and N 2s, 2p-orbital. This mean that the covalent contribution between metal ion and donor atoms differentiates the complex formation stabilities, leading to the Am
5f-orbital and N 2s, 2p-orbital. This mean that the covalent contribution between metal ion and donor atoms differentiates the complex formation stabilities, leading to the Am /Eu
/Eu selectivity. We expect that this study contributes to systematize the origin of metal ions selectivity and to accelerate novel ligands exploration.
selectivity. We expect that this study contributes to systematize the origin of metal ions selectivity and to accelerate novel ligands exploration.
Fukuda, Tatsuya*; Takahashi, Ryo*; Hara, Takuhi*; Ohara, Koji*; Kato, Kazuo*; Matsumura, Daiju; Inaba, Yusuke*; Nakase, Masahiko*; Takeshita, Kenji*
Journal of Nuclear Science and Technology, 58(4), p.399 - 404, 2021/04
Times Cited Count:6 Percentile:60.71(Nuclear Science & Technology)Okamura, Tomohiro*; Oizumi, Akito; Nishihara, Kenji; Nakase, Masahiko*; Takeshita, Kenji*
JAEA-Data/Code 2020-023, 32 Pages, 2021/03
Nuclear Material Balance code (NMB code) have been developed in Japan Atomic Energy Agency. The NMB code will be updated with the function of mass balance analysis at the backend process such as reprocessing, vitrification and geological disposal. In order to perform its analysis with high accuracy, it is necessary to expand the number of FP nuclides calculated in the NMB code. In this study, depletion calculation by ORIGEN code was performed under 3 different burn-up conditions such as spent uranium fuel from light water reactor, and nuclides were selected from 5 evaluation indexes such as mass and heat generation. In addition, the FP nuclides required to configure a simple burnup chain with the same calculation accuracy as ORIGEN in the NMB code was selected. As the result, two lists with different number of nuclides, such as "Detailed list" and a "Simplified list", were created.
Okamura, Tomohiro*; Oizumi, Akito; Nishihara, Kenji; Nakase, Masahiko*; Takeshita, Kenji*
Bulletin of the Laboratory for Advanced Nuclear Energy, 5, P. 31, 2021/02
The Takeshita Laboratory at Tokyo Institute of Technology has started to develop a Nuclear Material Balance code (NMB code) in collaboration with Japan Atomic Energy Agency. This report summarized the results of the joint research conducted in 2019.
Matsumiya, Masahiko*; Tsuchida, Yusuke*; Sasaki, Yuji; Ono, Ryoma*; Nakase, Masahiko*; Takeshita, Kenji*
Journal of Radioanalytical and Nuclear Chemistry, 327(1), p.597 - 607, 2021/01
Times Cited Count:2 Percentile:24.28(Chemistry, Analytical)To achieve trichotomic separation of light lanthanides (Ln), heavy Ln, and Am, batchwise multi-stage extractions using tetraoctyl-diglycolamide (TODGA) extractant from organic acids are studied. Malonic acid (MA) has high solubility in water and is used as the main component of the aqueous phase. It is clear that the separation factor (SF) for Nd/Am from MA and that for La/Am from MA + HNO are both around 30. The light Ln (e.g., La and Ce) flowed-out in 1 M MA+0.05 M HNO
are both around 30. The light Ln (e.g., La and Ce) flowed-out in 1 M MA+0.05 M HNO (1st soln.), Am is recovered into 3 M MA (2nd soln.), and middle and heavy Ln (Nd and other heavy Ln) are back-extracted into 0.1 M TEDGA/water (3rd soln.). This extraction method can give 95% recovery of Am with total Ln of less than 16% present in high-level radioactive waste.
(1st soln.), Am is recovered into 3 M MA (2nd soln.), and middle and heavy Ln (Nd and other heavy Ln) are back-extracted into 0.1 M TEDGA/water (3rd soln.). This extraction method can give 95% recovery of Am with total Ln of less than 16% present in high-level radioactive waste.
Sasaki, Yuji; Nakase, Masahiko*
Petorotekku, 43(11), p.782 - 787, 2020/11
As analog compounds of DGA (diglycolamide), MIDOA(methylimino-diacetamide) and TDGA(thia-diglycolammide) are used for the extractants of platinum group metals. These extractants can be extracted noble metals and oxyanions, which followed by HSAB theory. The high concentration of these metals can be also extracted by these compounds. The research of metal-complex structures gives the information on the ability and role for complex-formation, which will be useful for the development of novel extractants.
 -tetraoctyl-diglycolamide from organic acid
-tetraoctyl-diglycolamide from organic acidSasaki, Yuji; Matsumiya, Masahiko*; Nakase, Masahiko*; Takeshita, Kenji*
Chemistry Letters, 49(10), p.1216 - 1219, 2020/10
Times Cited Count:9 Percentile:45.29(Chemistry, Multidisciplinary)Lanthanide (Ln) extractions from organic acids to  -dodecane by
-dodecane by  -tetraoctyl-diglycolamide (TODGA) were conducted. Four organic acids (lactic acid, malonic acid, tartaric acid, and citric acid) were employed. Although these acids stabilize lanthanides in the aqueous phase, a distribution ratio (
-tetraoctyl-diglycolamide (TODGA) were conducted. Four organic acids (lactic acid, malonic acid, tartaric acid, and citric acid) were employed. Although these acids stabilize lanthanides in the aqueous phase, a distribution ratio ( ) greater 1 was obtained for heavy Ln. Ln patterns (
) greater 1 was obtained for heavy Ln. Ln patterns ( (Ln) against atomic number of Ln) show maximum values of Ho and Er. In order to obtain high
(Ln) against atomic number of Ln) show maximum values of Ho and Er. In order to obtain high  values, the addition of HNO
values, the addition of HNO in aqueous phase is found to be effective.
in aqueous phase is found to be effective.
 system
systemSasaki, Yuji; Morita, Keisuke; Matsumiya, Masahiko*; Nakase, Masahiko*
Radiochimica Acta, 108(9), p.689 - 699, 2020/09
Times Cited Count:9 Percentile:75.92(Chemistry, Inorganic & Nuclear)The simultaneous separation of Am and Cm from lanthanides is important for atomic energy fields. All lanthanides, Am, and Cm can be extracted by diglycolamide (DGA). In addition, relatively high separation factors between An and Ln were obtained by the extraction system of TODGA, DTPA (diethylenetriamine-pentaacetic acid) and HNO . In this work, DTPA-BA (diethylenetriamine-triacetic-bisamide), which is an improved version of DTPA, was employed for the separation of Ln and An. A relatively high separation factor (approximately 8) for actinides/lanthanides was obtained. Then, the multi-step extraction was performed. Thus, the recoveries of 94.7% for Nd and 4.7% for Am and Cm in organic phase, and 5.3% Nd and 95.3% for Am and Cm in aqueous phase were obtained.
. In this work, DTPA-BA (diethylenetriamine-triacetic-bisamide), which is an improved version of DTPA, was employed for the separation of Ln and An. A relatively high separation factor (approximately 8) for actinides/lanthanides was obtained. Then, the multi-step extraction was performed. Thus, the recoveries of 94.7% for Nd and 4.7% for Am and Cm in organic phase, and 5.3% Nd and 95.3% for Am and Cm in aqueous phase were obtained.
Tsutsui, Nao; Ban, Yasutoshi; Suzuki, Hideya*; Nakase, Masahiko*; Ito, Sayumi*; Inaba, Yusuke*; Matsumura, Tatsuro; Takeshita, Kenji*
Analytical Sciences, 36(2), p.241 - 246, 2020/02
Times Cited Count:21 Percentile:79.8(Chemistry, Analytical)To investigate the effective separation of actinides (Ans) from lanthanides (Lns), single-stage batch extraction experiments were performed with a novel extractant, tetradodecyl-1,10-phenanthroline-2,9-diamide (TDdPTDA) with various diluents such as 3-nitrobenzotrifluoride (F-3), nitrobenzene, and  -dodecane for Am, Cm, and Lns. The extraction kinetics with TDdPTDA was rapid enough to perform the actual extraction flow sheet. The slopes of the distribution ratio versus TDdPTDA concentration and the distribution ratio versus nitric acid concentration were similar for F-3 and nitrobenzene systems but different from
-dodecane for Am, Cm, and Lns. The extraction kinetics with TDdPTDA was rapid enough to perform the actual extraction flow sheet. The slopes of the distribution ratio versus TDdPTDA concentration and the distribution ratio versus nitric acid concentration were similar for F-3 and nitrobenzene systems but different from  -dodecane system. These differences were attributed to the characteristics of the diluents. This study reveals high distribution ratios of Am (
-dodecane system. These differences were attributed to the characteristics of the diluents. This study reveals high distribution ratios of Am (
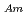 ) and Cm (
) and Cm (
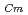 ) for TDdPTDA, with the high separation factors (
) for TDdPTDA, with the high separation factors ( s) of Am from Lns enough for their separation.
s) of Am from Lns enough for their separation.
Mishima, Ria; Inaba, Yusuke*; Tachioka, Sotaro*; Harigai, Miki*; Watanabe, Shinta*; Onoe, Jun*; Nakase, Masahiko*; Matsumura, Tatsuro; Takeshita, Kenji*
Chemistry Letters, 49(1), p.83 - 86, 2020/01
Times Cited Count:4 Percentile:21.18(Chemistry, Multidisciplinary)Separation of platinum group metals (PGMs) from high-level liquid waste generated from the reprocessing of spent nuclear fuels is important to produce good quality vitrified glass for final disposal. A new sorbent, Aluminum hexacyanoferrate (AlHCF), was synthesized and the general sorption behavior of PGMs from concentrated nitric acid was examined. Nitric acid caused substantial elution of AlHCF but the sorption of Pd stabilized the structure. Consequently, Rh was sorbed in the presence of Pd, whereas single Rh sorption caused complete dissolution of AlHCF. Relation between sorbed mount of Pd vs eluted Al and Fe revealed that the elution ratio of Al and Fe was not the same as molar ratio of synthesized AlHCF, indicating the re-sorption of Fe resulted in formation of new structure. The sorption mechanism of PGMs by this new sorbent, AlHCF, not only the simple ion exchange, but also oxidation reduction reaction as well as kinetics play important rule. Understanding the general sorption and dissolution behavior will help improve the sorption performance of PGMs by AlHCF.
Sasaki, Yuji; Morita, Keisuke; Matsumiya, Masahiko*; Nakase, Masahiko*
Proceedings of International Nuclear Fuel Cycle Conference / Light Water Reactor Fuel Performance Conference (Global/Top Fuel 2019) (USB Flash Drive), p.108 - 112, 2019/09
We attempted to separate An from Ln, and Am and Cm by the system including extractant and masking agent. The separation factor of Nd and Am was approximately 10 by TODGA-DTPA-BA and that of Am and Cm was over 3 by TODGA-DOODA(C2). Using these batch data, profiles of metal concentration with multi-step extractions proposed in this manuscript were demonstrated.