Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Miyagawa, Akihisa*; Hayashi, Naoki*; Iwamoto, Hibiki*; Arai, Tsuyoshi*; Nagatomo, Shigenori*; Miyazaki, Yasunori; Hasegawa, Kenta; Sano, Yuichi; Nakatani, Kiyoharu*
Analytical Sciences, 40(2), p.347 - 352, 2024/02
Times Cited Count:2 Percentile:23.37(Chemistry, Analytical)The Eu(III) distribution mechanism in single extractant-impregnated polymer-layered silica particle in a complex solution containing multiple lanthanide ions was investigated using fluorescence microspectroscopy. The rate-determining step was the reaction of Eu(III) with the two extractant molecules. The obtained mechanism and rate constants agreed with those of the single-ion distribution system, in which Eu(III) was distributed to the particles in the Eu(III) solution.
 -tetraoctyldiglycolamide-impregnated polymer-coated silica particle using fluorescence microspectroscopy; Transfer mechanism and effect of polymer crosslinking degree
-tetraoctyldiglycolamide-impregnated polymer-coated silica particle using fluorescence microspectroscopy; Transfer mechanism and effect of polymer crosslinking degreeMiyagawa, Akihisa*; Takahashi, Takumi*; Kuzure, Yoshiaki*; Iwamoto, Hibiki*; Arai, Tsuyoshi*; Nagatomo, Shigenori*; Watanabe, So; Sano, Yuichi; Nakatani, Kiyoharu*
Analytical Sciences, 39(11), p.1929 - 1936, 2023/11
Times Cited Count:1 Percentile:10.71(Chemistry, Analytical)In this study, we revealed the Eu(III) distribution in a single diglycolamide-derivative extractant (TODGA)-impregnated polymer-coated silica particle. The reaction of Eu(III) with two TODGA molecules in the polymer layer was the rate-limiting process, as evidenced by the absence of any correlation between the rate constants (k and k
and k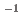 ) and concentrations of Eu(III) and HNO
) and concentrations of Eu(III) and HNO .
.
Miyagawa, Akihisa*; Hayashi, Naoki*; Iwamoto, Hibiki*; Arai, Tsuyoshi*; Nagatomo, Shigenori*; Miyazaki, Yasunori; Hasegawa, Kenta; Sano, Yuichi; Nakatani, Kiyoharu*
Bulletin of the Chemical Society of Japan, 96(9), p.1019 - 1025, 2023/09
Times Cited Count:4 Percentile:41.44(Chemistry, Multidisciplinary)In the present study, we have elucidated the mass transfer mechanism of Eu(III) and Sm(III) in the solution with these ions in single nitrilotriacetamide (NTA) extractant-impregnated polymer-coated silica particle. The rate-limiting process of mass transfer was the reaction process of ions with NTA molecules, in which the NO
 ions were not involved, which was consistent with that obtained in single ion distribution system.
ions were not involved, which was consistent with that obtained in single ion distribution system.
Miyagawa, Akihisa*; Hayashi, Naoki*; Kuzure, Yoshiaki*; Takahashi, Takumi*; Iwamoto, Hibiki*; Arai, Tsuyoshi*; Nagatomo, Shigenori*; Miyazaki, Yasunori; Hasegawa, Kenta; Sano, Yuichi; et al.
Bulletin of the Chemical Society of Japan, 96(7), p.671 - 676, 2023/07
Times Cited Count:8 Percentile:61.41(Chemistry, Multidisciplinary)We investigated the distribution mechanism of Eu(III) in a single polymer-coated silica particle including nitrilotriacetamide (NTA) extractants known as HONTA and TOD2EHNTA. The present study provides a valuable approach for the evaluation and enhancement of the functionality of "single extractant-impregnated polymer-coated silica particle".
 aqueous solution system revealed by fluorescence microspectroscopy
aqueous solution system revealed by fluorescence microspectroscopyMiyagawa, Akihisa*; Kusano, Yuka*; Nagatomo, Shigenori*; Sano, Yuichi; Nakatani, Kiyoharu*
Analytical Sciences, 38(7), p.955 - 961, 2022/07
Times Cited Count:1 Percentile:5.53(Chemistry, Analytical)In this study, we reveal an Eu(III) extraction mechanism at the interface between HNO and tributyl phosphate (TBP) solutions using fluorescence microspectroscopy. The mass transfer rate constant at the interface is obtained from the analysis of fluorescence intensity changes during the forward and backward extractions at various HNO
and tributyl phosphate (TBP) solutions using fluorescence microspectroscopy. The mass transfer rate constant at the interface is obtained from the analysis of fluorescence intensity changes during the forward and backward extractions at various HNO and TBP concentrations to investigate the reaction mechanism. This result indicates that one nitrate ion reacts with Eu(III) at the interface, whereas TBP molecules are not involved in the interfacial reaction, which is different from the results obtained using the NaNO
and TBP concentrations to investigate the reaction mechanism. This result indicates that one nitrate ion reacts with Eu(III) at the interface, whereas TBP molecules are not involved in the interfacial reaction, which is different from the results obtained using the NaNO solution in our previous study. We demonstrate that the chemical species of Eu(III) complex with nitrate ion and TBP in the aqueous solution play an important role for the extraction mechanism.
solution in our previous study. We demonstrate that the chemical species of Eu(III) complex with nitrate ion and TBP in the aqueous solution play an important role for the extraction mechanism.
Sano, Yuichi; Sakamoto, Atsushi; Miyazaki, Yasunori; Watanabe, So; Morita, Keisuke; Emori, Tatsuya; Ban, Yasutoshi; Arai, Tsuyoshi*; Nakatani, Kiyoharu*; Matsuura, Haruaki*; et al.
Proceedings of International Conference on Nuclear Fuel Cycle; Sustainable Energy Beyond the Pandemic (GLOBAL 2022) (Internet), 4 Pages, 2022/07
We developed a hybrid MA(III) recovery process combining MA(III)+Ln(III) co-recovery flowsheet by solvent extraction with TBP and MA(III)/Ln(III) separation flowsheet by simulated moving bed chromatography using HONTA impregnated adsorbents with large particle size porous silica support.
Miyagawa, Akihisa*; Takeuchi, Masayuki; Arai, Tsuyoshi*; Watanabe, So; Sano, Yuichi; Nakatani, Kiyoharu*
Bulletin of the Chemical Society of Japan, 95(4), p.566 - 568, 2022/04
Times Cited Count:3 Percentile:20.54(Chemistry, Multidisciplinary)We demonstrate that pKa of extractant impregnated in a polymer phase varies with the cross-linking degree and the coexistence of other extractants, which induces a change in the hydrophobicity of the polymer phase. The results presented herein will be beneficial for the development of novel solid-extraction adsorbents.
Watanabe, So; Senzaki, Tatsuya; Shibata, Atsuhiro; Nomura, Kazunori; Takeuchi, Masayuki; Nakatani, Kiyoharu*; Matsuura, Haruaki*; Horiuchi, Yusuke*; Arai, Tsuyoshi*
Journal of Radioanalytical and Nuclear Chemistry, 322(3), p.1273 - 1277, 2019/12
Times Cited Count:6 Percentile:47.17(Chemistry, Analytical)Otaka, Toshiki*; Sato, Tatsumi*; Ono, Shimpei; Nagoshi, Kohei; Abe, Ryoji*; Arai, Tsuyoshi*; Watanabe, So; Sano, Yuichi; Takeuchi, Masayuki; Nakatani, Kiyoharu*
Analytical Sciences, 35(10), p.1129 - 1133, 2019/10
Times Cited Count:9 Percentile:33.12(Chemistry, Analytical)Sano, Yuichi; Arai, Tsuyoshi*; Nakatani, Kiyoharu*; Matsuura, Haruaki*; Kunii, Shigeru*
no journal, ,
JAEA has developed a hybrid process combining solvent extraction and low pressure loss extraction chromatography to achieve a reasonable MA(III) recovery process having superior safety and economy. In the solvent extraction, MA(III) is recovered with Ln(III) using inexpensive TBP extractants and centrifugal contactors having superior phase separation performance. In the extraction chromatography, MA(III) is separated from Ln(III) using specially designed adsorbents for low pressure loss. In this report, the outline of R&D program will be introduced.
Watanabe, So; Senzaki, Tatsuya; Shibata, Atsuhiro; Nomura, Kazunori; Nakatani, Kiyoharu*; Horiuchi, Yusuke*; Arai, Tsuyoshi*; Matsuura, Haruaki*
no journal, ,
no abstracts in English
Kusano, Yuka*; Miyagawa, Akihisa*; Nagatomo, Shigenori*; Nakatani, Kiyoharu*; Sano, Yuichi
no journal, ,
The solvent extraction / back-extraction process of europium with tributyl phosphate was kinetically analyzed from the microfluorescence measurement of micro organic droplets. From the rate constant dependence on nitric acid, nitrate ion, and tributyl phosphate concentrations, it was clarified that extraction is governed by the movement of europium with two nitrate ions at the organic-aqueous interface, and the quantitative relationship between the inverse extraction rate and these concentrations.
Watanabe, So; Senzaki, Tatsuya; Shibata, Atsuhiro; Nomura, Kazunori; Takeuchi, Masayuki; Nakatani, Kiyoharu*; Matsuura, Haruaki*; Horiuchi, Yusuke*; Arai, Tsuyoshi*
no journal, ,
no abstracts in English
Sano, Yuichi; Arai, Tsuyoshi*; Nakatani, Kiyoharu*; Matsuura, Haruaki*; Kunii, Shigeru*
no journal, ,
A MA(III)+Ln(III) co-recovery flowsheet by solvent extraction using TBP and a MA(III)/Ln(III) separation flowsheet by simulated moving bed (SMB) chromatography using HONTA impregnated adsorbent with a large particle size porous silica support that enables low pressure drop operation were designed. The proposed process was confirmed to have advantages in terms of waste generation, safety, and economics compared to previously proposed processes consisting only of solvent extraction or extraction chromatography.
Hayashi, Naoki*; Miyagawa, Akihisa*; Nagatomo, Shigenori*; Nakatani, Kiyoharu*; Sato, Kiyomori*; Arai, Tsuyoshi*; Ambai, Hiromu; Hasegawa, Kenta; Watanabe, So; Sano, Yuichi
no journal, ,
The fluorescence of lanthanide ions within a single extractant-impregnated silica particle was obtained using a microspectrometer to evaluate their distribution behavior within the particle.
Kusano, Yuka*; Nakagawa, Ryogo*; Nagatomo, Shigenori*; Sano, Yuichi; Nakatani, Kiyoharu*
no journal, ,
The columnar cavities formed on the microelectrodes were filled with a tributyl phosphate extractant, immersed in a nitric acid solution, and irradiated with laser light on the columnar droplets, and the temporal changes in the luminescence intensity of the lanthanoid complex were tracked. The change with time was analyzed by a single exponential function, and the extraction and back-extraction rates were calculated. The mechanism of mass transfer was discussed kinetically from the viewpoints of diffusion inside and outside the droplet, interfacial reaction, etc.
Sano, Yuichi; Arai, Tsuyoshi*; Nakatani, Kiyoharu*; Matsuura, Haruaki*; Kunii, Shigeru*
no journal, ,
We have been developing a hybrid process combining solvent extraction and low pressure loss extraction chromatography for recovering trivalent minor actinides (MA(III): Am, Cm), which will decrease the waste and have good safety and economic performances. In this presentation, the outline of R&D program and some recent results will be introduced.
Otaka, Toshiki*; Sato, Tatsumi*; Nakatani, Kiyoharu*; Nagoshi, Kohei*; Abe, Ryoji*; Arai, Tsuyoshi*; Watanabe, So; Sano, Yuichi; Takeuchi, Masayuki
no journal, ,
no abstracts in English
Kusano, Yuka*; Miyagawa, Akihisa*; Nagatomo, Shigenori*; Nakatani, Kiyoharu*; Sano, Yuichi
no journal, ,
We measured the concentration dependencies of TBP, nitric acid, and nitrate ion for extraction and back extraction rates of FP (Ln) in a single microdroplet/solution system by fluorescence microspectroscopy technique, and determined mass transfer coefficients at the oil/water interfaces. The mass transfer coefficients, influenced significantly by those concentrations, will be discussed in detail.
Kuzure, Yoshiaki*; Miyagawa, Akihisa*; Nagatomo, Shigenori*; Nakatani, Kiyoharu*; Yoshida, Masaaki*; Nemoto, Shuhei*; Arai, Tsuyoshi*; Ambai, Hiromu; Hasegawa, Kenta; Sano, Yuichi
no journal, ,
Concentration dependencies of nitric acid, nitrate ion, and so on for the adsorption and elution rates (mass transfer coefficients) of FP (Ln) in silica adsorbent, carrying various NTAamide extractants, were measured in the single silica particle/solution system by fluorescence microspectroscopy. The adsorption and elution rates were improved by changing polymercoating conditions for the silica adsorbent granulated by the freeze-drying method. We will discuss the mass transfer coefficients influenced by the nitric acid and nitrate ionconcentrations.