Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Yoshida, Maiko; Honda, Mitsuru; Narita, Emi*; Hayashi, Nobuhiko; Urano, Hajime; Nakata, Motoki; Miyato, Naoaki; Takenaga, Hidenobu; Ide, Shunsuke; Kamada, Yutaka
Nuclear Fusion, 55(7), p.073014_1 - 073014_9, 2015/07
Times Cited Count:15 Percentile:56.33(Physics, Fluids & Plasmas)Conditions without the increases in the thermal and particle transport with ECH have been experimentally investigated in positive magnetic shear (PS), weak magnetic shear (WS) and reversed magnetic shear (RS) plasmas with internal transport barriers (ITBs) on JT-60U. The ion heat diffusivity around an internal transport barrier in the ion temperature (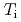 -ITB) remains constant with ECH when a large negative toroidal rotation shear is formed before the ECH. The condition does not depend on the electron to ion temperature ratio (
-ITB) remains constant with ECH when a large negative toroidal rotation shear is formed before the ECH. The condition does not depend on the electron to ion temperature ratio (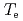 /
/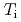 ) and ECH power. The electron heat diffusivity around a
) and ECH power. The electron heat diffusivity around a 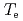 -ITB stays constant with ECH when the magnetic shear is negative around the Te-ITB region. Effective particle transport remains constant or reduces during ECH under the condition of negative magnetic shear.
-ITB stays constant with ECH when the magnetic shear is negative around the Te-ITB region. Effective particle transport remains constant or reduces during ECH under the condition of negative magnetic shear.
Narita, Emi*; Honda, Mitsuru; Hayashi, Nobuhiko; Urano, Hajime; Ide, Shunsuke; Fukuda, Takeshi*
Plasma and Fusion Research (Internet), 10, p.1403019_1 - 1403019_11, 2015/03
Garcia, J.*; Hayashi, Nobuhiko; Baiocchi, B.*; Giruzzi, G.*; Honda, Mitsuru; Ide, Shunsuke; Maget, P.*; Narita, Emi*; Schneider, M.*; Urano, Hajime; et al.
Nuclear Fusion, 54(9), p.093010_1 - 093010_13, 2014/09
Times Cited Count:42 Percentile:86.00(Physics, Fluids & Plasmas)Narita, Emi; Honda, Mitsuru; Hayashi, Nobuhiko; Takizuka, Tomonori*; Ide, Shunsuke; Itami, Kiyoshi; Isayama, Akihiko; Fukuda, Takeshi*
Plasma and Fusion Research (Internet), 8, p.1403082_1 - 1403082_8, 2013/06

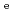 /
/
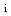 effect on confinement properties
effect on confinement propertiesNarita, Emi*; Takizuka, Tomonori*; Hayashi, Nobuhiko; Fujita, Takaaki; Ide, Shunsuke; Honda, Mitsuru; Isayama, Akihiko; Itami, Kiyoshi; Kamada, Yutaka; Tanaka, Yasuyuki*; et al.
Plasma and Fusion Research (Internet), 7(Sp.1), p.2403102_1 - 2403102_5, 2012/07
Yamamoto, Yoshihisa*; Togashi, Hideaki*; Kato, Atsushi*; Suemitsu, Maki*; Narita, Yuzuru*; Teraoka, Yuden; Yoshigoe, Akitaka
Materials Research Society Symposium Proceedings, Vol.1074, p.36 - 40, 2008/00
In this study, we have investigated the bonding structure of ultrathin oxide films on Si(110) surface by real-time SR-PES experiments. Experiments were conducted at surface chemistry end-station settled at BL23SU in SPring-8. The oxidation temperature was 813 K and the O pressure was 1.1
pressure was 1.1 10
10 Pa. As a result, we found that one of the surface core-level shifts in Si 2p spectrum, related to the 1st and the 2nd layer Si atoms, decreases rapidly, coincident with the rapid initial development of the O1s spectrum. This indicates high reactivity of the Si(110)-16
Pa. As a result, we found that one of the surface core-level shifts in Si 2p spectrum, related to the 1st and the 2nd layer Si atoms, decreases rapidly, coincident with the rapid initial development of the O1s spectrum. This indicates high reactivity of the Si(110)-16 2 reconstructed surface with oxygen molecules.
2 reconstructed surface with oxygen molecules.
 2 surfaces by O 1s photoemission spectroscopy using synchrotron radiation
2 surfaces by O 1s photoemission spectroscopy using synchrotron radiationSuemitsu, Maki*; Kato, Atsushi*; Togashi, Hideaki*; Konno, Atsushi*; Yamamoto, Yoshihisa*; Teraoka, Yuden; Yoshigoe, Akitaka; Narita, Yuzuru*; Enta, Yoshiharu*
Japanese Journal of Applied Physics, Part 1, 46(4B), p.1888 - 1890, 2007/04
Times Cited Count:12 Percentile:43.55(Physics, Applied)Initial oxidation of Si(110) surface has been investigated by using real-time X-ray photoemission spectroscopy. The time evolution of the O 1s spectrum shows occurrence of rapid oxidation just after the introduction of the oxygen molecules, which is evidenced by the considerable peak intensity corresponding to oxygen exposure of as low as 1.5L (1L=1.33 10
10 Pa s). This initial oxide is dominated by a state with a relatively low binding energy, which is gradually replaced by a state with a relatively high binding energy with the increase of the oxygen exposure, resulting in the low-KE shift of the O 1s peak. Comparison with previously reported O 1s spectra from dry-oxidized Si(111) surface suggests oxidation at or around the adatoms of Si(110)-16
Pa s). This initial oxide is dominated by a state with a relatively low binding energy, which is gradually replaced by a state with a relatively high binding energy with the increase of the oxygen exposure, resulting in the low-KE shift of the O 1s peak. Comparison with previously reported O 1s spectra from dry-oxidized Si(111) surface suggests oxidation at or around the adatoms of Si(110)-16 2 clean surface as a likely oxidation state for this low-binding-energy peak.
2 clean surface as a likely oxidation state for this low-binding-energy peak.
Suemitsu, Maki*; Kato, Atsushi*; Togashi, Hideaki*; Konno, Atsushi*; Yamamoto, Yoshihisa*; Teraoka, Yuden; Yoshigoe, Akitaka; Narita, Yuzuru*
Shingaku Giho, 106(108), p.61 - 63, 2006/06
no abstracts in English
 2 surface by photoemission spectroscopy
2 surface by photoemission spectroscopySuemitsu, Maki*; Kato, Atsushi*; Togashi, Hideaki*; Konno, Atsushi*; Yamamoto, Yoshihisa*; Teraoka, Yuden; Yoshigoe, Akitaka; Enta, Yoshiharu*; Narita, Yuzuru*
ECS Transactions, 3(2), p.311 - 316, 2006/00
Initial thermal oxidation of Si(110) surface has been investigated by using real-time X-ray photoemission spectroscopy with synchrotron radiation. The Si(110) initial oxidation is characterized by presence of a rapid oxidation just after the introduction of gaseous oxygen molecules. Peak separation of the O1s photoemission spectra suggests the presence of at least two distinct oxidation sites on the surface, which may reflect the complicated surface structure of the Si(110)-16 2 reconstruction.
2 reconstruction.
Narita, Emi; Honda, Mitsuru; Hayashi, Nobuhiko; Urano, Hajime; Ide, Shunsuke; Fukuda, Takeshi*
no journal, ,
no abstracts in English
Suemitsu, Maki*; Kato, Atsushi*; Togashi, Hideaki*; Konno, Atsushi*; Yamamoto, Yoshihisa*; Teraoka, Yuden; Yoshigoe, Akitaka; Narita, Yuzuru*
no journal, ,
Initial kinetics in dry oxidation of Si(110) surface, a key technology in the next-generation CMOS technology, has been investigated by using synchrotron-radiation photoemission spectroscopy. As a result, the uptake curve of the Si(110) oxide in its high-pressure-low-temperature reaction regime is found to consist of three characteristic time domains, in sharp contrast with the single domain obtained on Si(001). The difference is understood in terms of the different atomistic arrangement on the surface.
Suemitsu, Maki*; Kato, Atsushi*; Togashi, Hideaki*; Konno, Atsushi*; Yamamoto, Yoshihisa*; Teraoka, Yuden; Yoshigoe, Akitaka; Narita, Yuzuru*
no journal, ,
no abstracts in English
 2 surface using synchrotron radiation photoelectron spectroscopy
2 surface using synchrotron radiation photoelectron spectroscopyKato, Atsushi*; Togashi, Hideaki*; Yamamoto, Yoshihisa*; Konno, Atsushi*; Narita, Yuzuru*; Suemitsu, Maki*; Teraoka, Yuden; Yoshigoe, Akitaka; Takahashi, Yuya*; Asaoka, Hidehito
no journal, ,
no abstracts in English
 2 surface and its interface structure
2 surface and its interface structureYamamoto, Yoshihisa*; Togashi, Hideaki*; Kato, Atsushi*; Suemitsu, Maki*; Narita, Yuzuru*; Teraoka, Yuden; Yoshigoe, Akitaka
no journal, ,
We have investigated oxidation at Si(110) surfaces via real-time photoemission spectroscopy with synchrotron radiation. We have already obtained the following information that rapid initial oxidation region was in the early stage of oxidation and one of shifted Si2p core level components was decreased with increasing oxide. Therefore, we focused our interest to the topmost ultrathin oxide-layer formation and investigated time evolution of oxide components of Si2p photoemission spectrum. All experiments were conducted at surface chemistry station of BL23SU in the SPring-8. The oxygen pressure was 1.0 10
10 Pa and substrate temperature was 813 K. Consequently, concerning oxide components of Si2p during oxidation, the intensity of Si
Pa and substrate temperature was 813 K. Consequently, concerning oxide components of Si2p during oxidation, the intensity of Si component was larger than that of Si
component was larger than that of Si until 1 ML oxidation.
until 1 ML oxidation.
Yamamoto, Yoshihisa*; Togashi, Hideaki*; Kato, Atsushi*; Suemitsu, Maki*; Narita, Yuzuru*; Teraoka, Yuden; Yoshigoe, Akitaka
no journal, ,
Formation processes of one atomic oxide layer and time evolution of chemical bonding states at the interface were investigated by using real-time photoemission spectroscopy with synchrotron radiation. Time evolutions for oxide components (Si :n=1-4) of Si2p photoemission spectra, observed in the conditions of 813K substrate temperature and 1.1
:n=1-4) of Si2p photoemission spectra, observed in the conditions of 813K substrate temperature and 1.1 10
10 Pa O
Pa O pressure, were analyzed. Rapid increase of Si
pressure, were analyzed. Rapid increase of Si component until oxygen dose of 10 L indicates existance of a reaction path through the Si
component until oxygen dose of 10 L indicates existance of a reaction path through the Si state. On the other hand, contrary to the Si(001) oxidation, the Si
state. On the other hand, contrary to the Si(001) oxidation, the Si component was larger than the Si
component was larger than the Si component during oxidation. Reaction mechanisms was considered from these facts. Oxygen insertion into chain-like Si-Si bonds densely existing on the (110) surface (A bond) and Si-Si bonds on the (-110) surface is an origin of the first layer oxidation at Si(110) surface. The A bonds may be oxidized partially due to storage of oxidation strains.
component during oxidation. Reaction mechanisms was considered from these facts. Oxygen insertion into chain-like Si-Si bonds densely existing on the (110) surface (A bond) and Si-Si bonds on the (-110) surface is an origin of the first layer oxidation at Si(110) surface. The A bonds may be oxidized partially due to storage of oxidation strains.
Narita, Emi; Takizuka, Tomonori*; Hayashi, Nobuhiko; Ide, Shunsuke; Honda, Mitsuru; Fukuda, Takeshi*
no journal, ,
no abstracts in English
Narita, Emi; Honda, Mitsuru; Hayashi, Nobuhiko; Urano, Hajime; Ide, Shunsuke; Fukuda, Takeshi*
no journal, ,
no abstracts in English
Garcia, J.*; Hayashi, Nobuhiko; Baiocchi, B.*; Citrin, J.*; Giruzzi, G.*; Honda, Mitsuru; Ide, Shunsuke; Maget, P.*; Narita, Emi*; Schneider, M.*; et al.
no journal, ,
Hayashi, Nobuhiko; Garcia, J.*; Narita, Emi
no journal, ,
Narita, Emi*; Honda, Mitsuru; Hayashi, Nobuhiko; Urano, Hajime; Ide, Shunsuke; Fukuda, Takeshi*
no journal, ,