Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Narita, Hirokazu*; Maeda, Motoki*; Tokoro, Chiharu*; Suzuki, Tomoya*; Tanaka, Mikiya*; Shiwaku, Hideaki; Yaita, Tsuyoshi
RSC Advances (Internet), 13(25), p.17001 - 17007, 2023/06
Times Cited Count:3 Percentile:32.03(Chemistry, Multidisciplinary)no abstracts in English
Suzuki, Tomoya*; Otsubo, Ukyo*; Ogata, Takeshi*; Shiwaku, Hideaki; Kobayashi, Toru; Yaita, Tsuyoshi; Matsuoka, Mitsuaki*; Murayama, Norihiro*; Narita, Hirokazu*
Separation and Purification Technology, 308, p.122943_1 - 122943_7, 2023/03
Times Cited Count:3 Percentile:15.39(Engineering, Chemical)HNO leaching is used in recycling Pd metal from spent products that primarily contain Ag, and most Pd residues are separated from solutions containing Ag(I). However, a small amount of Pd(II) often remains in these Ag(I) solutions. Therefore, the separation of Pd(II) and Ag(I) in HNO
leaching is used in recycling Pd metal from spent products that primarily contain Ag, and most Pd residues are separated from solutions containing Ag(I). However, a small amount of Pd(II) often remains in these Ag(I) solutions. Therefore, the separation of Pd(II) and Ag(I) in HNO solutions is essential to promote efficient Pd recycling. In this study, the separation of Pd(II) and Ag(I) in HNO
solutions is essential to promote efficient Pd recycling. In this study, the separation of Pd(II) and Ag(I) in HNO solutions was investigated using four N-donor-type adsorbents functionalized with amine (R-Amine), iminodiacetic acid (R-IDA), pyridine (R-Py), or bis-picolylamine (R-BPA). R-Amine, R-IDA, and R-Py selectively adsorbed Pd(II) over Ag(I), Cu(II), Ni(II), and Fe(III) from HNO
solutions was investigated using four N-donor-type adsorbents functionalized with amine (R-Amine), iminodiacetic acid (R-IDA), pyridine (R-Py), or bis-picolylamine (R-BPA). R-Amine, R-IDA, and R-Py selectively adsorbed Pd(II) over Ag(I), Cu(II), Ni(II), and Fe(III) from HNO solutions (0.3-7 M), but R-Amine exhibited a lower Pd adsorption efficiency. In contrast,
solutions (0.3-7 M), but R-Amine exhibited a lower Pd adsorption efficiency. In contrast,  90% of Pd(II), Ag(I), and Cu(II) were adsorbed by R-BPA over the entire range of HNO
90% of Pd(II), Ag(I), and Cu(II) were adsorbed by R-BPA over the entire range of HNO concentrations. Structural analyses of the adsorbed metal ions using Fourier transform infrared spectroscopy and extended X-ray absorption fine structure spectroscopy revealed the separation mechanisms of the N-donor-type adsorbents. Pd(II) adsorption on R-IDA, R-Py, and R-BPA occurred via Pd(II) coordination of the functional groups (iminodiacetic acid, pyridine, and bis-picolylamine, respectively), whereas that on R-Amine occurred via anion exchange of NO
concentrations. Structural analyses of the adsorbed metal ions using Fourier transform infrared spectroscopy and extended X-ray absorption fine structure spectroscopy revealed the separation mechanisms of the N-donor-type adsorbents. Pd(II) adsorption on R-IDA, R-Py, and R-BPA occurred via Pd(II) coordination of the functional groups (iminodiacetic acid, pyridine, and bis-picolylamine, respectively), whereas that on R-Amine occurred via anion exchange of NO
 with [Pd(NO
with [Pd(NO )
) ]
] . The coordinative adsorption mechanisms resulted in the higher Pd(II) adsorption behaviors of R-IDA, R-Py, and R-BPA. HCl (5.0 M) and thiourea (0.1 M) eluents desorbed 83% of Pd(II) from R-IDA and 95% from R-Py, respectively. R-Py was the most effective Pd(II) adsorbent based on adsorption selectivity and desorption efficiency.
. The coordinative adsorption mechanisms resulted in the higher Pd(II) adsorption behaviors of R-IDA, R-Py, and R-BPA. HCl (5.0 M) and thiourea (0.1 M) eluents desorbed 83% of Pd(II) from R-IDA and 95% from R-Py, respectively. R-Py was the most effective Pd(II) adsorbent based on adsorption selectivity and desorption efficiency.
Uchino, Seiko*; Narita, Hirokazu*; Kita, Keisuke*; Suzuki, Hideya*; Matsumura, Tatsuro; Naganawa, Hirochika*; Sakaguchi, Koichi*; Oto, Keisuke*
Solvent Extraction Research and Development, Japan, 30(1), p.39 - 46, 2023/00
The extraction of trivalent rare earth ions (RE ) from HNO
) from HNO solution using a triamide amine, tris(N,N-di-2-ethylhexyl-ethylamide)amine (DEHTAA), was conducted, and the extraction mechanism was estimated from extraction behavior of HNO
solution using a triamide amine, tris(N,N-di-2-ethylhexyl-ethylamide)amine (DEHTAA), was conducted, and the extraction mechanism was estimated from extraction behavior of HNO and RE
and RE and the relationship between atomic number and extraction percentages (E%) for RE
and the relationship between atomic number and extraction percentages (E%) for RE . A DEHTAA molecule dominantly formed a DEHTAA HNO
. A DEHTAA molecule dominantly formed a DEHTAA HNO at 1.0 M HNO
at 1.0 M HNO and a DEHTAA(HNO
and a DEHTAA(HNO )
) at 6.0 M HNO
at 6.0 M HNO in the acid-equilibrated organic phase. This would provide the unique dependence of E% for the light RE
in the acid-equilibrated organic phase. This would provide the unique dependence of E% for the light RE on the HNO
on the HNO concentration, in which the E% value had a minimum and maximum at
concentration, in which the E% value had a minimum and maximum at  0.5 M and
0.5 M and  2 M HNO
2 M HNO , respectively. The results of the slope analyses for the distribution ratios for RE
, respectively. The results of the slope analyses for the distribution ratios for RE suggested that the dominant RE
suggested that the dominant RE complex was RE(NO
complex was RE(NO )
) DEHTAA(DEHTAA HNO
DEHTAA(DEHTAA HNO ) at 1.0 M HNO
) at 1.0 M HNO . The E% for RE
. The E% for RE decreased from La
decreased from La to Lu
to Lu at 1.0 M HNO
at 1.0 M HNO ; on the other hand, those increased from La
; on the other hand, those increased from La to Nd
to Nd at 0.25 M and from La
at 0.25 M and from La to Sm
to Sm and 6.0 M HNO
and 6.0 M HNO .
.
Akutsu-Suyama, Kazuhiro*; Yamada, Norifumi*; Ueda, Yuki; Motokawa, Ryuhei; Narita, Hirokazu*
Applied Sciences (Internet), 12(3), p.1215_1 - 1215_10, 2022/02
Times Cited Count:6 Percentile:59.69(Chemistry, Multidisciplinary)no abstracts in English
Suzuki, Tomoya*; Otsubo, Ukyo*; Ogata, Takeshi*; Shiwaku, Hideaki; Kobayashi, Toru; Yaita, Tsuyoshi; Matsuoka, Mitsuaki*; Murayama, Norihiro*; Narita, Hirokazu*
Dalton Transactions (Internet), 50(33), p.11390 - 11397, 2021/09
Times Cited Count:4 Percentile:29.31(Chemistry, Inorganic & Nuclear)no abstracts in English
Nakano, Masanao; Fujii, Tomoko; Nemoto, Masashi; Tobita, Keiji; Seya, Natsumi; Nishimura, Shusaku; Hosomi, Kenji; Nagaoka, Mika; Yokoyama, Hiroya; Matsubara, Natsumi; et al.
JAEA-Review 2020-069, 163 Pages, 2021/02
Environmental radiation monitoring around the Tokai Reprocessing Plant has been performed by the Nuclear Fuel Cycle Engineering Laboratories, based on "Safety Regulations for the Reprocessing Plant of Japan Atomic Energy Agency, Chapter IV - Environmental Monitoring". This annual report presents the results of the environmental monitoring and the dose estimation to the hypothetical inhabitant due to the radioactivity discharged from the plant to the atmosphere and the sea during April 2019 to March 2020. In this report, some data include the influence of the accidental release from the Fukushima Daiichi Nuclear Power Station of Tokyo Electric Power Co., Inc. (the trade name was changed to Tokyo Electric Power Company Holdings, Inc. on April 1, 2016) in March 2011. Appendices present comprehensive information, such as monitoring programs, monitoring methods, monitoring results and their trends, meteorological data and discharged radioactive wastes. In addition, the data which were influenced by the accidental release and exceeded the normal range of fluctuation in the monitoring, were evaluated.
Narita, Hirokazu*; Kasuya, Ryo*; Suzuki, Tomoya*; Motokawa, Ryuhei; Tanaka, Mikiya*
Encyclopedia of Inorganic and Bioinorganic Chemistry (Internet), 28 Pages, 2020/12
Suzuki, Tomoya*; Ogata, Takeshi*; Tanaka, Mikiya*; Kobayashi, Toru; Shiwaku, Hideaki; Yaita, Tsuyoshi; Narita, Hirokazu*
Analytical Sciences, 35(12), p.1353 - 1360, 2019/12
Times Cited Count:5 Percentile:17.62(Chemistry, Analytical)no abstracts in English
Narita, Hirokazu*; Nicolson, R. M.*; Motokawa, Ryuhei; Ito, Fumiyuki*; Morisaku, Kazuko*; Goto, Midori*; Tanaka, Mikiya*; Heller, W. T.*; Shiwaku, Hideaki; Yaita, Tsuyoshi; et al.
Inorganic Chemistry, 58(13), p.8720 - 8734, 2019/07
Times Cited Count:21 Percentile:75.49(Chemistry, Inorganic & Nuclear) -trimethylglycine
-trimethylglycineSuzuki, Tomoya*; Narita, Hirokazu*; Ogata, Takeshi*; Suzuki, Hideya; Matsumura, Tatsuro; Kobayashi, Toru; Shiwaku, Hideaki; Yaita, Tsuyoshi
Solvent Extraction Research and Development, Japan, 26(1), p.11 - 19, 2019/06
Times Cited Count:3 Percentile:11.39(Chemistry, Multidisciplinary)The ability of AMP03, a styrene-divinylbenzene copolymer functionalized with  -trimethylglycine moieties, to adsorb Pd(II) from HNO
-trimethylglycine moieties, to adsorb Pd(II) from HNO solutions was investigated to elucidate the affinity of
solutions was investigated to elucidate the affinity of  -trimethylglycine for Pd(II). In the present study, we investigated the mechanism of Pd(II) adsorption by AMP03 by means of adsorption experiments, Fourier Transform Infrared (FT-IR) spectroscopy, and Extended X-ray Absorption Fine Structure (EXAFS) spectroscopy.
-trimethylglycine for Pd(II). In the present study, we investigated the mechanism of Pd(II) adsorption by AMP03 by means of adsorption experiments, Fourier Transform Infrared (FT-IR) spectroscopy, and Extended X-ray Absorption Fine Structure (EXAFS) spectroscopy.
Suzuki, Tomoya*; Ogata, Takeshi*; Tanaka, Mikiya*; Kobayashi, Toru; Shiwaku, Hideaki; Yaita, Tsuyoshi; Narita, Hirokazu*
Metals, 8(7), p.558_1 - 558_10, 2018/07
Times Cited Count:18 Percentile:59.10(Materials Science, Multidisciplinary)The refining of platinum group metals is based mainly on solvent extraction methods, whereas Ru is selectively recovered by distillation as RuO . Replacement of distillation byextraction is expected to simplify the purification process. To develop an effective extraction system for Ru, we analyzed the Ru species in HCl with UV-Vis and EXAFS spectroscopies, and we examined the properties of Ru extracted with N-2-ethylhexyl-bis(N-di-2-ethylhexyl-ethylamide) amine (EHBAA). EXAFS and UV-Vis spectra of Ru in HCl solutions revealed that the predominant Ru species in 0.5-10 M HCl solutions changed from [RuCl
. Replacement of distillation byextraction is expected to simplify the purification process. To develop an effective extraction system for Ru, we analyzed the Ru species in HCl with UV-Vis and EXAFS spectroscopies, and we examined the properties of Ru extracted with N-2-ethylhexyl-bis(N-di-2-ethylhexyl-ethylamide) amine (EHBAA). EXAFS and UV-Vis spectra of Ru in HCl solutions revealed that the predominant Ru species in 0.5-10 M HCl solutions changed from [RuCl (H
(H O)
O) ]
] to [RuCl
to [RuCl ]
]
 with the HCl concentration. The extraction percentages of Ru in the EHBAA system increased with increasing HCl concentration, reached 80% at [HCl] = 5 M, and decreased athigher HCl concentrations. EXAFS analysis of the extracted complex indicated that the Ru
with the HCl concentration. The extraction percentages of Ru in the EHBAA system increased with increasing HCl concentration, reached 80% at [HCl] = 5 M, and decreased athigher HCl concentrations. EXAFS analysis of the extracted complex indicated that the Ru
 had 5 Cl
had 5 Cl and 1 H
and 1 H O in its inner coordination sphere. The similarity of the dependence on HCl concentrations of the extraction in the EHBAA system and the distribution profile of [RuCl
O in its inner coordination sphere. The similarity of the dependence on HCl concentrations of the extraction in the EHBAA system and the distribution profile of [RuCl (H
(H O)]
O)]
 on [RuCl
on [RuCl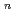 (H
(H O)
O)
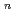 ]
]

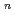 suggested that the EHBAA extracted the pentachlorido species.
suggested that the EHBAA extracted the pentachlorido species.
Narita, Hirokazu*; Maeda, Motoki*; Tokoro, Chiharu*; Suzuki, Tomoya*; Tanaka, Mikiya*; Motokawa, Ryuhei; Shiwaku, Hideaki; Yaita, Tsuyoshi
Analytical Sciences, 33(11), p.1305 - 1309, 2017/11
Times Cited Count:11 Percentile:36.51(Chemistry, Analytical)Narita, Hirokazu*; Suzuki, Tomoya*; Motokawa, Ryuhei
Nihon Kinzoku Gakkai-Shi, 81(4), p.157 - 167, 2017/04
Times Cited Count:16 Percentile:55.22(Metallurgy & Metallurgical Engineering)Maeda, Motoki*; Narita, Hirokazu*; Tokoro, Chiharu*; Tanaka, Mikiya*; Motokawa, Ryuhei; Shiwaku, Hideaki; Yaita, Tsuyoshi
Separation and Purification Technology, 177, p.176 - 181, 2017/04
Times Cited Count:22 Percentile:55.11(Engineering, Chemical)Motokawa, Ryuhei; Kobayashi, Toru; Endo, Hitoshi*; Ikeda, Takashi; Yaita, Tsuyoshi; Suzuki, Shinichi; Narita, Hirokazu*; Akutsu, Kazuhiro*; Heller, W. T.*
Journal of Nuclear Science and Technology, 53(8), p.1205 - 1211, 2016/08
Times Cited Count:1 Percentile:9.49(Nuclear Science & Technology)Narita, Hirokazu*; Tanaka, Mikiya*; Sato, Yumiko*; Yaita, Tsuyoshi; Okamoto, Yoshihiro
Solvent Extraction and Ion Exchange, 24(5), p.693 - 702, 2006/05
Times Cited Count:14 Percentile:46.66(Chemistry, Multidisciplinary)The structure of the Ni(II) complex extracted with the commercial hydroxyoxime, LIX84I, and the effect of adding bis(2-ethylhexyl)phosphoricacid(D2EHPA) to LIX84I on the extraction rate and the coordination of Ni(II) were investigated by solvent extraction and XAFS methods. The XANES spectrum and the curve fit soft the EXAFS spectrum of the Ni-LIX84I complex showed that the complex is four-coordinate square-planar with a 1:2 stoichiometry. In the Ni(II)-D2EHPA-LIX84I system, the coordination geometry changes to six-coordinate octahedral with an increase in the D2EHPA concentration. Although the rate of Ni(II) extraction with LIX84I is significantly accelerated by adding as small amount of D2EHPA, most of the Ni(II) complexes extracted with this organic solution remain square-planar. This indicates that the effect of D2EHPA on the increase in the extraction rate would be attributed to a behavior of this ligand like catalysis.
 solutions using
solutions using  -dimethyl-
-dimethyl- -diphenylpyridine-2,6-dicarboxyamide
-diphenylpyridine-2,6-dicarboxyamideShimada, Asako; Yaita, Tsuyoshi; Narita, Hirokazu; Tachimori, Shoichi; Kimura, Takaumi; Okuno, Kenji*;
Solvent Extraction Research and Development, Japan, 11, p.1 - 10, 2004/04
The distribution ratio ( ) of Am(III) and lighter Ln(III) in the extraction with
) of Am(III) and lighter Ln(III) in the extraction with  -dimethyl-
-dimethyl- -diphenylpyridine-2,6-dicarboxyamide(DMDPhPDA) from HNO
-diphenylpyridine-2,6-dicarboxyamide(DMDPhPDA) from HNO solutions were determined. The D's increased with an increase of HNO
solutions were determined. The D's increased with an increase of HNO concentration. Additionally, separation of Am(III) from Ln(III) were confirmed for all the HNO
concentration. Additionally, separation of Am(III) from Ln(III) were confirmed for all the HNO concentration range (S.F.=(D
concentration range (S.F.=(D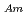 /D
/D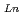 )). From 4 M HNO
)). From 4 M HNO solution with 0.5 M DMDPhPDA CHCl
solution with 0.5 M DMDPhPDA CHCl solution, the
solution, the  's of Am(III), Eu(III) and Nd(III) were 1.3, 0.25 and 0.24, respectively. This result suggests that Am(III) can be separated from Eu and Nd in the higher HNO
's of Am(III), Eu(III) and Nd(III) were 1.3, 0.25 and 0.24, respectively. This result suggests that Am(III) can be separated from Eu and Nd in the higher HNO concentration region than that has been reported so far. These
concentration region than that has been reported so far. These  's value and the S.F. were reproduced in the extraction from the metal concentration range from 10
's value and the S.F. were reproduced in the extraction from the metal concentration range from 10 M order to 10
M order to 10 M.
M.
 -dimethyl-
-dimethyl- -diphenylpyridine-2,6-dicarboxyamide(DMDPhPDA) from nitric acid solutions
-diphenylpyridine-2,6-dicarboxyamide(DMDPhPDA) from nitric acid solutionsShimada, Asako; Yaita, Tsuyoshi; Narita, Hirokazu; Tachimori, Shoichi; Okuno, Kenji*
Solvent Extraction and Ion Exchange, 22(2), p.147 - 161, 2004/03
Times Cited Count:68 Percentile:81.34(Chemistry, Multidisciplinary)The distribution ratios of lanthanides with  -dimethyl-
-dimethyl- -diphenylpyridine-2,6-dicarboxyamide(DMDPhPDA) from 1-5M nitric acid solutions were determined and the extraction mechanism was discussed on the basis of the slope analyses of acid and ligand concentration dependencies and lanthanide patterns (variation of the distribution ratio as a function of ionic radius). The lanthanide extractions were explained through two mechanisms from the standpoint of the formation of the extracted complex as follows: (1) the formation of inner-sphere complex with two DMDPhPDA molecules for light lanthanides in the extraction from HNO
-diphenylpyridine-2,6-dicarboxyamide(DMDPhPDA) from 1-5M nitric acid solutions were determined and the extraction mechanism was discussed on the basis of the slope analyses of acid and ligand concentration dependencies and lanthanide patterns (variation of the distribution ratio as a function of ionic radius). The lanthanide extractions were explained through two mechanisms from the standpoint of the formation of the extracted complex as follows: (1) the formation of inner-sphere complex with two DMDPhPDA molecules for light lanthanides in the extraction from HNO less than 3M, and (2) the formation of outer-sphere complex with the third DMDPhPDA molecules in addition to the inner-sphere complex in the extraction of light lanthanides from HNO
less than 3M, and (2) the formation of outer-sphere complex with the third DMDPhPDA molecules in addition to the inner-sphere complex in the extraction of light lanthanides from HNO more than 3M, and those of heavy lanthanides from 1-5M HNO
more than 3M, and those of heavy lanthanides from 1-5M HNO solutions. Nitric acid concentration is more influential than the ligand concentration in the formation of outer-sphere complex.
solutions. Nitric acid concentration is more influential than the ligand concentration in the formation of outer-sphere complex.
 by K-absorption edge XAFS
by K-absorption edge XAFSOkamoto, Yoshihiro; Shiwaku, Hideaki; Yaita, Tsuyoshi; Narita, Hirokazu; Tanida, Hajime*
Journal of Molecular Structure, 641(1), p.71 - 76, 2002/10
Times Cited Count:43 Percentile:71.81(Chemistry, Physical)The local structure of molten LaCl was investigated by X-ray absorption fine structure(XAFS) of the La K-edge. The nearest La
was investigated by X-ray absorption fine structure(XAFS) of the La K-edge. The nearest La -Cl
-Cl distance andcoordination number were 2.89
distance andcoordination number were 2.89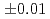
 and 7.4
and 7.4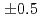 from the curve fitting of the 1st peak in the fourier transform magnitude
from the curve fitting of the 1st peak in the fourier transform magnitude 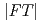 . The coordination number larger than 6 suggests that the local structure of molten LaCl
. The coordination number larger than 6 suggests that the local structure of molten LaCl is not a simple octahedral coordination (LaCl
is not a simple octahedral coordination (LaCl )
) , but 7-fold (LaCl
, but 7-fold (LaCl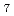 )
) and/or 8-fold (LaCl
and/or 8-fold (LaCl )
)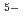 complexes. The 1st La
complexes. The 1st La -La
-La distance, of which correlation was observed as a weak 2nd peak in the
distance, of which correlation was observed as a weak 2nd peak in the 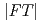 , was evaluated to be 4.9
, was evaluated to be 4.9 . It suggests that the distorted corner-sharing connection of the complex species is predominant in the melt, inontrast with molten YCl
. It suggests that the distorted corner-sharing connection of the complex species is predominant in the melt, inontrast with molten YCl in which the edge-sharing connection of the 6-fold (YCl
in which the edge-sharing connection of the 6-fold (YCl )
) mainly exists.
mainly exists.
Narita, Hirokazu; Yaita, Tsuyoshi; Suzuki, Shinichi; Takai, Konomi; Tachimori, Shoichi; Motohashi, Haruhiko*
Journal of Synchrotron Radiation, 8(Part2), p.672 - 673, 2001/03
no abstracts in English