Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Suzuki, Tomoya*; Otsubo, Ukyo*; Ogata, Takeshi*; Shiwaku, Hideaki; Kobayashi, Toru; Yaita, Tsuyoshi; Matsuoka, Mitsuaki*; Murayama, Norihiro*; Narita, Hirokazu*
Separation and Purification Technology, 308, p.122943_1 - 122943_7, 2023/03
Times Cited Count:2 Percentile:24.43(Engineering, Chemical)HNO leaching is used in recycling Pd metal from spent products that primarily contain Ag, and most Pd residues are separated from solutions containing Ag(I). However, a small amount of Pd(II) often remains in these Ag(I) solutions. Therefore, the separation of Pd(II) and Ag(I) in HNO
leaching is used in recycling Pd metal from spent products that primarily contain Ag, and most Pd residues are separated from solutions containing Ag(I). However, a small amount of Pd(II) often remains in these Ag(I) solutions. Therefore, the separation of Pd(II) and Ag(I) in HNO solutions is essential to promote efficient Pd recycling. In this study, the separation of Pd(II) and Ag(I) in HNO
solutions is essential to promote efficient Pd recycling. In this study, the separation of Pd(II) and Ag(I) in HNO solutions was investigated using four N-donor-type adsorbents functionalized with amine (R-Amine), iminodiacetic acid (R-IDA), pyridine (R-Py), or bis-picolylamine (R-BPA). R-Amine, R-IDA, and R-Py selectively adsorbed Pd(II) over Ag(I), Cu(II), Ni(II), and Fe(III) from HNO
solutions was investigated using four N-donor-type adsorbents functionalized with amine (R-Amine), iminodiacetic acid (R-IDA), pyridine (R-Py), or bis-picolylamine (R-BPA). R-Amine, R-IDA, and R-Py selectively adsorbed Pd(II) over Ag(I), Cu(II), Ni(II), and Fe(III) from HNO solutions (0.3-7 M), but R-Amine exhibited a lower Pd adsorption efficiency. In contrast,
solutions (0.3-7 M), but R-Amine exhibited a lower Pd adsorption efficiency. In contrast,  90% of Pd(II), Ag(I), and Cu(II) were adsorbed by R-BPA over the entire range of HNO
90% of Pd(II), Ag(I), and Cu(II) were adsorbed by R-BPA over the entire range of HNO concentrations. Structural analyses of the adsorbed metal ions using Fourier transform infrared spectroscopy and extended X-ray absorption fine structure spectroscopy revealed the separation mechanisms of the N-donor-type adsorbents. Pd(II) adsorption on R-IDA, R-Py, and R-BPA occurred via Pd(II) coordination of the functional groups (iminodiacetic acid, pyridine, and bis-picolylamine, respectively), whereas that on R-Amine occurred via anion exchange of NO
concentrations. Structural analyses of the adsorbed metal ions using Fourier transform infrared spectroscopy and extended X-ray absorption fine structure spectroscopy revealed the separation mechanisms of the N-donor-type adsorbents. Pd(II) adsorption on R-IDA, R-Py, and R-BPA occurred via Pd(II) coordination of the functional groups (iminodiacetic acid, pyridine, and bis-picolylamine, respectively), whereas that on R-Amine occurred via anion exchange of NO
 with [Pd(NO
with [Pd(NO )
) ]
] . The coordinative adsorption mechanisms resulted in the higher Pd(II) adsorption behaviors of R-IDA, R-Py, and R-BPA. HCl (5.0 M) and thiourea (0.1 M) eluents desorbed 83% of Pd(II) from R-IDA and 95% from R-Py, respectively. R-Py was the most effective Pd(II) adsorbent based on adsorption selectivity and desorption efficiency.
. The coordinative adsorption mechanisms resulted in the higher Pd(II) adsorption behaviors of R-IDA, R-Py, and R-BPA. HCl (5.0 M) and thiourea (0.1 M) eluents desorbed 83% of Pd(II) from R-IDA and 95% from R-Py, respectively. R-Py was the most effective Pd(II) adsorbent based on adsorption selectivity and desorption efficiency.
Suzuki, Tomoya*; Otsubo, Ukyo*; Ogata, Takeshi*; Shiwaku, Hideaki; Kobayashi, Toru; Yaita, Tsuyoshi; Matsuoka, Mitsuaki*; Murayama, Norihiro*; Narita, Hirokazu*
Dalton Transactions (Internet), 50(33), p.11390 - 11397, 2021/09
Times Cited Count:2 Percentile:21.56(Chemistry, Inorganic & Nuclear)no abstracts in English
Suzuki, Tomoya*; Ogata, Takeshi*; Tanaka, Mikiya*; Kobayashi, Toru; Shiwaku, Hideaki; Yaita, Tsuyoshi; Narita, Hirokazu*
Analytical Sciences, 35(12), p.1353 - 1360, 2019/12
Times Cited Count:3 Percentile:12.45(Chemistry, Analytical)no abstracts in English
 -trimethylglycine
-trimethylglycineSuzuki, Tomoya*; Narita, Hirokazu*; Ogata, Takeshi*; Suzuki, Hideya; Matsumura, Tatsuro; Kobayashi, Toru; Shiwaku, Hideaki; Yaita, Tsuyoshi
Solvent Extraction Research and Development, Japan, 26(1), p.11 - 19, 2019/06
Times Cited Count:3 Percentile:14.02(Chemistry, Multidisciplinary)The ability of AMP03, a styrene-divinylbenzene copolymer functionalized with  -trimethylglycine moieties, to adsorb Pd(II) from HNO
-trimethylglycine moieties, to adsorb Pd(II) from HNO solutions was investigated to elucidate the affinity of
solutions was investigated to elucidate the affinity of  -trimethylglycine for Pd(II). In the present study, we investigated the mechanism of Pd(II) adsorption by AMP03 by means of adsorption experiments, Fourier Transform Infrared (FT-IR) spectroscopy, and Extended X-ray Absorption Fine Structure (EXAFS) spectroscopy.
-trimethylglycine for Pd(II). In the present study, we investigated the mechanism of Pd(II) adsorption by AMP03 by means of adsorption experiments, Fourier Transform Infrared (FT-IR) spectroscopy, and Extended X-ray Absorption Fine Structure (EXAFS) spectroscopy.
Suzuki, Tomoya*; Ogata, Takeshi*; Tanaka, Mikiya*; Kobayashi, Toru; Shiwaku, Hideaki; Yaita, Tsuyoshi; Narita, Hirokazu*
Metals, 8(7), p.558_1 - 558_10, 2018/07
Times Cited Count:12 Percentile:53.94(Materials Science, Multidisciplinary)The refining of platinum group metals is based mainly on solvent extraction methods, whereas Ru is selectively recovered by distillation as RuO . Replacement of distillation byextraction is expected to simplify the purification process. To develop an effective extraction system for Ru, we analyzed the Ru species in HCl with UV-Vis and EXAFS spectroscopies, and we examined the properties of Ru extracted with N-2-ethylhexyl-bis(N-di-2-ethylhexyl-ethylamide) amine (EHBAA). EXAFS and UV-Vis spectra of Ru in HCl solutions revealed that the predominant Ru species in 0.5-10 M HCl solutions changed from [RuCl
. Replacement of distillation byextraction is expected to simplify the purification process. To develop an effective extraction system for Ru, we analyzed the Ru species in HCl with UV-Vis and EXAFS spectroscopies, and we examined the properties of Ru extracted with N-2-ethylhexyl-bis(N-di-2-ethylhexyl-ethylamide) amine (EHBAA). EXAFS and UV-Vis spectra of Ru in HCl solutions revealed that the predominant Ru species in 0.5-10 M HCl solutions changed from [RuCl (H
(H O)
O) ]
] to [RuCl
to [RuCl ]
]
 with the HCl concentration. The extraction percentages of Ru in the EHBAA system increased with increasing HCl concentration, reached 80% at [HCl] = 5 M, and decreased athigher HCl concentrations. EXAFS analysis of the extracted complex indicated that the Ru
with the HCl concentration. The extraction percentages of Ru in the EHBAA system increased with increasing HCl concentration, reached 80% at [HCl] = 5 M, and decreased athigher HCl concentrations. EXAFS analysis of the extracted complex indicated that the Ru
 had 5 Cl
had 5 Cl and 1 H
and 1 H O in its inner coordination sphere. The similarity of the dependence on HCl concentrations of the extraction in the EHBAA system and the distribution profile of [RuCl
O in its inner coordination sphere. The similarity of the dependence on HCl concentrations of the extraction in the EHBAA system and the distribution profile of [RuCl (H
(H O)]
O)]
 on [RuCl
on [RuCl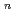 (H
(H O)
O)
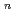 ]
]

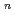 suggested that the EHBAA extracted the pentachlorido species.
suggested that the EHBAA extracted the pentachlorido species.
Narita, Emi*; Honda, Mitsuru; Hayashi, Nobuhiko; Urano, Hajime; Ide, Shunsuke; Fukuda, Takeshi*
Plasma and Fusion Research (Internet), 10, p.1403019_1 - 1403019_11, 2015/03
Narita, Emi; Honda, Mitsuru; Hayashi, Nobuhiko; Takizuka, Tomonori*; Ide, Shunsuke; Itami, Kiyoshi; Isayama, Akihiko; Fukuda, Takeshi*
Plasma and Fusion Research (Internet), 8, p.1403082_1 - 1403082_8, 2013/06

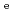 /
/
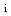 effect on confinement properties
effect on confinement propertiesNarita, Emi*; Takizuka, Tomonori*; Hayashi, Nobuhiko; Fujita, Takaaki; Ide, Shunsuke; Honda, Mitsuru; Isayama, Akihiko; Itami, Kiyoshi; Kamada, Yutaka; Tanaka, Yasuyuki*; et al.
Plasma and Fusion Research (Internet), 7(Sp.1), p.2403102_1 - 2403102_5, 2012/07
Otsuka, Satoshi; Kaito, Takeji; Inoue, Masaki; Asayama, Tai; Kim, S.-W.; Ukai, Shigeharu*; Narita, Takeshi*; Sakasegawa, Hideo*
Journal of Nuclear Materials, 386-388, p.479 - 482, 2009/04
Times Cited Count:19 Percentile:76.21(Materials Science, Multidisciplinary)This paper discusses the effects of small portion of Al contamination ( 0.1wt%) on the high-temperature strength and microstructure of 9Cr-ODS steel. Increasing Al concentration is shown to provide small reduction of ultimate tensile strength as well as 0.2% proof stress at 973 K and 1073 K accompanied by reduction of elongated grains i.e. residual-
0.1wt%) on the high-temperature strength and microstructure of 9Cr-ODS steel. Increasing Al concentration is shown to provide small reduction of ultimate tensile strength as well as 0.2% proof stress at 973 K and 1073 K accompanied by reduction of elongated grains i.e. residual- ferrite acting as reinforcement phase. Addition of Al appears to increase the proportion of ferrite phase, which is contrary to general behavior in conventional steels. This unique behavior could be peculiar to the non-equilibrium materials such as mechanically-alloyed alloy. Computer simulation on phase transformation suggests that the fine oxide dispersion in the elongated ferrite could be attributable to the preferential partitioning of Ti and W in ferrite than in austenite at hot-extrusion process at 1423 K.
ferrite acting as reinforcement phase. Addition of Al appears to increase the proportion of ferrite phase, which is contrary to general behavior in conventional steels. This unique behavior could be peculiar to the non-equilibrium materials such as mechanically-alloyed alloy. Computer simulation on phase transformation suggests that the fine oxide dispersion in the elongated ferrite could be attributable to the preferential partitioning of Ti and W in ferrite than in austenite at hot-extrusion process at 1423 K.
Narita, Takeshi; Ukai, Shigeharu; Kaito, Takeji; Otsuka, Satoshi; Matsuda, Yasushi*
Journal of Nuclear Science and Technology, 45(2), p.99 - 102, 2008/02
Times Cited Count:5 Percentile:35.07(Nuclear Science & Technology)The oxide dispersion strengthened (ODS) ferritic-martensitic steels are being developing for application as advanced fast reactor cladding and fusion blanket materials, in order to allow increased operation temperature. Water corrosion test of ODS ferritic-martensitic steels was conducted under a controlled alkali water environment to evaluate the water corrosion behavior, comparing to conventional 17 mass% Cr austenitic stainless steel (PNC316) and 11 mass% Cr ferritic-martensitic stainless steel (PNC-FMS). It was showed that 9Cr-ODS martensitic steel and 12Cr-ODS ferritic steel have superior water corrosion resistance, and comparable to that of PNC316 and PNC-FMS at 333K for 1,000h under varying pH of 8.4, 10, 12.
Otsuka, Satoshi; Ukai, Shigeharu; Sakasegawa, Hideo; Fujiwara, Masayuki; Kaito, Takeji; Narita, Takeshi
Journal of Nuclear Materials, 367-370(1), p.160 - 165, 2007/08
Times Cited Count:59 Percentile:95.87(Materials Science, Multidisciplinary)This paper describes the effect on creep strength and microstructure of 9Cr-oxide dispersion strengthened martensitic steel (9Cr-ODS steel) brought by the differences in titanium concentration and consolidation temperature. The increase of titanium concentration to 0.30-0.35wt% was shown to provide remarkable improvement of creep strength accompanied by the increase of residual-alpha ferrite. The elevation of hot-extrusion temperature notably degraded the creep strength, however, appeared to increase the volume fraction of residual-alpha ferrite. Creep deformation process of 9Cr-ODS steel was discussed to explain these results based on microstructure observations.
Matsuoka, Toshiyuki; Semba, Takeshi; Ishigaki, Koichi; Sugimoto, Yoshihiro*; Tanoue, Masayoshi*; Narita, Norifumi*
Nihon Oyo Chishitsu Gakkai Heisei-18-Nendo Kenkyu Happyokai Koen Rombunshu, p.331 - 334, 2006/11
no abstracts in English
Narita, Takeshi*; Ukai, Shigeharu; Kaito, Takeji; Otsuka, Satoshi; Fujiwara, Masayuki
JAEA-Research 2006-050, 85 Pages, 2006/10
In 9Cr ODS martensitic steel, tungsten(W) is a solid solution strengthening element, whose addition increases high-temperature strength by the combined effect with oxide dispersion strengthening. However, its excessive addition results in the increase of ferrite phase causing precipitation of intermetallic compound (Laves phase) under high temperature irradiation condition and thus ductility degradation. The amount of W addition therefore should be as low as possible. In this report, the effects of W on microstructure and high temperature mechanical properties of 9Cr ODS martensitic steels were examined for obtaining insights into optimum W concentration in terms of high-temperature strength and ductility. The results obtained are as follows: (1)In the 9CrODS martensitic steel, addition of W exceeding 2mass% is shown to cause precipitation of Laves phase which degrades the ductility and fracture toughness. It can be said that the current specification of W concentration, i.e. 2mass%W, is appropriate. (2)Hardness and tensile strength is shown to increase with W concentration. This increase is caused by the increase of solid solution strengthening and residual-alpha ferrite. The retainment of residual-alpha ferrite is enhanced by the addition of W (ferrite former element). The improvement of tensile strength at 973K provided by the solid solution strengthening is shown to be equivalent to that provided by the retainment of residual-alpha ferrite. (3)It would be open task to explorer an improved alloy design concept, i.e. decrease of W as low as possible and increase of residual-alpha ferrite. The degradation of high-temperature strength by decreasing W addition can be made up by the increasing fraction of residual-alpha phase that is provided by reduction of austenite former elements and increasing addition of ferrite former elements.
Narita, Takeshi; Ukai, Shigeharu; Kaito, Takeji; Otsuka, Satoshi; Matsuda, Yasushi*
JAEA-Research 2006-048, 52 Pages, 2006/07
As a part of feasibility study of ODS steel cladding, its water corrosion resistance was examined under water pool condition. Although addition of Cr is effective for preventing water corrosion, excessive Cr addition leads to embrittlement due to the Cr-rich  ' precipitate formation. In the ODS steel developed by the Japan Atomic Energy Agency (JAEA), the Cr content is controlled in 9Cr-ODS martensite and 12Cr-ODS ferrite. In this study, water corrosion test was conducted for these ODS steels, and their results were compared with that of conventional austenitic stainless steel and ferritic-martensitic stainless steel. Following results were obtained in this study. (1) Corrosion rate of 9Cr-ODS martensitic and 12Cr-ODS ferritic steel are significantly small and no pitting was observed. Thus, these ODS steels have superior resistance for water corrosion under the condition of 60
' precipitate formation. In the ODS steel developed by the Japan Atomic Energy Agency (JAEA), the Cr content is controlled in 9Cr-ODS martensite and 12Cr-ODS ferrite. In this study, water corrosion test was conducted for these ODS steels, and their results were compared with that of conventional austenitic stainless steel and ferritic-martensitic stainless steel. Following results were obtained in this study. (1) Corrosion rate of 9Cr-ODS martensitic and 12Cr-ODS ferritic steel are significantly small and no pitting was observed. Thus, these ODS steels have superior resistance for water corrosion under the condition of 60 C and pH8
C and pH8 12. (2) It was showed that 9CR-ODS martensitic and 12Cr-ODS ferritic steel have comparable water corrosion resistance to that of PNC316 and PNC-FMS at 60
12. (2) It was showed that 9CR-ODS martensitic and 12Cr-ODS ferritic steel have comparable water corrosion resistance to that of PNC316 and PNC-FMS at 60 C for 1000h under varying pH of 8, 10. Water corrosion resistance of these alloys is slightly larger than that of PNC316 and PNC-FMS at pH12 without significant differenceof appearance and uneven condition.
C for 1000h under varying pH of 8, 10. Water corrosion resistance of these alloys is slightly larger than that of PNC316 and PNC-FMS at pH12 without significant differenceof appearance and uneven condition.
Narita, Takeshi; Ukai, Shigeharu; Kaito, Takeji; Otsuka, Satoshi; Matsuda, Yasushi*
JAEA-Research 2006-047, 100 Pages, 2006/07
In a feasibility study of ODS steel cladding, its high temperature oxidation resistance was evaluated. Although addition of Cr is effective for preventing high temperature oxidation, excessively higher amount of Cr leads to embrittlement due to the Cr-rich  ' precipitate formation. In the ODS steel developed by the Japan Atomic Energy Agency (JAEA), the Cr content is controlled in 9Cr-ODS martensite and 12Cr-ODS ferrite. In this study, high temperature oxidation test was conducted for ODS steels, and their results were compared with that of conventional austenitic stainless steel and ferritic-martensitic stainless steel. Following results were obtained in this study. (1)9Cr-ODS martensitic and 12Cr-ODS ferritic steel have superior high temperature oxidation resistance compared to 11mass%Cr PNC-FMS and even 17mass% SUS430 and equivalent to austenitic PNC316. (2)The superior oxidation resistance of ODS steel was attributed to earlier formation of the protective alpha-Cr
' precipitate formation. In the ODS steel developed by the Japan Atomic Energy Agency (JAEA), the Cr content is controlled in 9Cr-ODS martensite and 12Cr-ODS ferrite. In this study, high temperature oxidation test was conducted for ODS steels, and their results were compared with that of conventional austenitic stainless steel and ferritic-martensitic stainless steel. Following results were obtained in this study. (1)9Cr-ODS martensitic and 12Cr-ODS ferritic steel have superior high temperature oxidation resistance compared to 11mass%Cr PNC-FMS and even 17mass% SUS430 and equivalent to austenitic PNC316. (2)The superior oxidation resistance of ODS steel was attributed to earlier formation of the protective alpha-Cr O
O layer at the matrix and inner oxide scale interface. The grain size of ODS steel is finer than that of PNC-FMS, so the superior oxidation resistance of ODS steel can be attributed to the enhanced Cr-supplying rate throughout the accelerated grain boundary diffusion. Finely dispersed Y2O3 oxide particles in the ODS steel matrix may also stabilized the adherence between the protective alpha-Cr
layer at the matrix and inner oxide scale interface. The grain size of ODS steel is finer than that of PNC-FMS, so the superior oxidation resistance of ODS steel can be attributed to the enhanced Cr-supplying rate throughout the accelerated grain boundary diffusion. Finely dispersed Y2O3 oxide particles in the ODS steel matrix may also stabilized the adherence between the protective alpha-Cr O
O layer and the matrix.
layer and the matrix.
Kaito, Takeji; Ukai, Shigeharu; Otsuka, Satoshi; Narita, Takeshi
Proceedings of International Conference on Nuclear Energy System for Future Generation and Global Sustainability (GLOBAL 2005) (CD-ROM), 6 Pages, 2005/10
Oxide Dispersion Strengthened (ODS) ferritic steel has excellent swelling resistance and improved high-temperature strength, which are important properties for the fast reactor fuel cladding tube. Japan Nuclear Cycle Development Institute (JNC) has developed a method for microstructure control to disperse nano-sized oxide particles in equiaxed crystal grain and succeeded in producing high performance ODS ferritic steel cladding. The ring tensile and internal creep rupture strengths of the manufactured ODS ferritic claddings exhibited excellent performance far beyond that of conventional ferritic-martensitic steel (PNC-FMS) and austenitic steel (PNC316). Adequate ductility in hoop direction was also maintained. The feasibility study was also conducted for economically manufacturing process with capable of large-scale production. In order to confirm and demonstrate the ODS fuel pin integrity to high burnup conditions, the irradiation test in BOR-60 has been conducted under the framework of JNC-Russia FBR cycle cooperation and the irradiation test in JOYO is under the planning stage.
Otsuka, Satoshi; Ukai, Shigeharu; Fujiwara, Masayuki; Kaito, Takeji; Narita, Takeshi
JNC TN9400 2005-034, 197 Pages, 2005/08
Oxide dispersion strengthened(ODS) martensitic steel (9CrODS steel) has been identified as an attractive candidate for advanced fast reactor (FR) fuel cladding tube because of its superior high-temperature strength and radiation resistance. Our recent activities revealed that high-temperature strength of different lots of the cladding tubes is inconsistent each other, even though the same manufacturing process was applied to these tubes. This inconsistency means a critical problem that high-strength 9CrODS steel cladding tubes can not be manufactured reliably and consistently. In this report, a microstructure control technique for consistently and reliably manufacturing high-strength 9CrODS steel cladding tubes are examined based on a series of data concerning effect of excess oxygen concentration on high temperature strength and microstructure of 9CrODS steel.
Otsuka, Satoshi; Ukai, Shigeharu; Kaito, Takeji; Narita, Takeshi; Fujiwara, Masayuki
Materials Transactions, 46(3), 487 Pages, 2005/00
Times Cited Count:45 Percentile:87.63(Materials Science, Multidisciplinary)None
Narita, Takeshi; Ukai, Shigeharu; Otsuka, Satoshi; Kaito, Takeji; Matsuda, Yasushi*
8th Workshop on the Ultra-Steel, 0 Pages, 2004/07
High temperature creep and oxidation tests are conducted, since the high temperature strength and oxidation resistance for the ODS ferritic steel are required for its applicability.As a result, It is identified that 9Cr-ODS martensitic steel demonstrated adquate creep strength at 973K, further titanum addition significantly improved the creep strength in 9Cr-ODS martensitic steel, and 9Cr-ODS martensitic and 12Cr-ODS ferritic steels have superior high temperature oxidation resistance compared to PNC-FMS and SUS430.
Narita, Takeshi; Ukai, Shigeharu; Kaito, Takeji; Otsuka, Satoshi; Fujiwara, Masayuki
JNC TN9400 2004-011, 141 Pages, 2004/04
Mass production capability of ODS martensitic steel claddinghas been evaluated in the feasibility studies on commercialized fast reactor cycle system. In this study, Manufacturing the large scale mother tube which has a high degree of accuracy in size, has been successfully carried out using large scale hollow capsule. For reducing the manufacturing cost of the ODS steel claddings, manufacturing process of the mother tubes using a large scale hollow capsules is promising.