Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
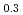 U
U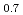 )O
)O
Nakamichi, Shinya; Hirooka, Shun; Kato, Masato; Sunaoshi, Takeo*; Nelson, A. T.*; McClellan, K. J.*
Journal of Nuclear Materials, 535, p.152188_1 - 152188_8, 2020/07
Times Cited Count:9 Percentile:75.92(Materials Science, Multidisciplinary)Oxygen-to-metal ratio (O/M) of uranium and plutonium mixed oxide depends on its oxygen partial pressure. To attain the desirable microstructure and O/M ratio of sintered pellets, it is important to investigate the relation between the sintering behavior and the atmosphere of sintering process. In this study, sintering behavior of (Pu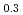 U
U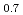 )O
)O and (Pu
and (Pu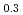 U
U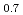 )O
)O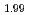 in controlled po
in controlled po atmosphere were investigated. It was found activation energy of (Pu
atmosphere were investigated. It was found activation energy of (Pu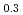 U
U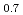 )O
)O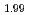 was higher than that of (Pu
was higher than that of (Pu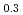 U
U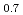 )O
)O . On the other hand, it was observed grain growth during sintering was suppressed in hypo-stoichiometric composition.
. On the other hand, it was observed grain growth during sintering was suppressed in hypo-stoichiometric composition.
Al-Shayeb, B.*; Sachdeva, R.*; Chen, L.-X.*; Ward, F.*; Munk, P.*; Devoto, A.*; Castelle, C. J.*; Olm, M. R.*; Bouma-Gregson, K.*; Amano, Yuki; et al.
Nature, 578(7795), p.425 - 431, 2020/02
Times Cited Count:220 Percentile:99.5(Multidisciplinary Sciences)
Suzuki, Kiichi; Kato, Masato; Sunaoshi, Takeo*; Uno, Hiroki*; Carvajal-Nunez, U.*; Nelson, A. T.*; McClellan, K. J.*
Journal of the American Ceramic Society, 102(4), p.1994 - 2008, 2019/04
Times Cited Count:36 Percentile:90.42(Materials Science, Ceramics)The fundamental properties of CeO were assessed using a range of experimental techniques. The oxygen potential of CeO
were assessed using a range of experimental techniques. The oxygen potential of CeO was measured by the thermogravimetric technique, and a numerical fit for the oxygen potential of CeO
was measured by the thermogravimetric technique, and a numerical fit for the oxygen potential of CeO is derived based on defect chemistry. Mechanical properties of CeO
is derived based on defect chemistry. Mechanical properties of CeO were obtained using sound velocity measurement, resonant ultrasound spectroscopy and nanoindentation. The obtained mechanical properties of CeO
were obtained using sound velocity measurement, resonant ultrasound spectroscopy and nanoindentation. The obtained mechanical properties of CeO are then used to evaluate the Debye temperature and Gruneisen constant. The heat capacity and thermal conductivity of CeO
are then used to evaluate the Debye temperature and Gruneisen constant. The heat capacity and thermal conductivity of CeO were also calculated using the Debye temperature and the Gruneisen constant. Finally, the thermal conductivity was calculated based upon laser flash analysis measurements. This result demonstrates that the thermal conductivity has strong dependence upon material purity.
were also calculated using the Debye temperature and the Gruneisen constant. Finally, the thermal conductivity was calculated based upon laser flash analysis measurements. This result demonstrates that the thermal conductivity has strong dependence upon material purity.
 in oxygen partial pressure-controlled atmosphere
in oxygen partial pressure-controlled atmosphereKato, Masato; Ikusawa, Yoshihisa; Sunaoshi, Takeo*; Nelson, A. T.*; McClellan, K. J.*
Journal of Nuclear Materials, 469, p.223 - 227, 2016/02
Times Cited Count:11 Percentile:71.62(Materials Science, Multidisciplinary)Thermal expansion of (U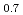 Pu
Pu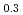 )O
)O (x = 0, 0.01, 0.02, 0.03) and (U
(x = 0, 0.01, 0.02, 0.03) and (U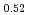 Pu
Pu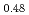 )O
)O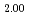 was measured with a dilatometer in an oxygen partial pressure-controlled atmosphere. The oxygen partial pressure was controlled to hold a constant oxygen-to-metal ratio in the (U,Pu)O
was measured with a dilatometer in an oxygen partial pressure-controlled atmosphere. The oxygen partial pressure was controlled to hold a constant oxygen-to-metal ratio in the (U,Pu)O during the measurement. Thermal expansion slightly increased with the decrease in oxygen-to-metal ratio. The relationship was derived to describe thermal expansion.
during the measurement. Thermal expansion slightly increased with the decrease in oxygen-to-metal ratio. The relationship was derived to describe thermal expansion.
 and (U,Pu)O
and (U,Pu)O
Nakamichi, Shinya; Hirooka, Shun; Sunaoshi, Takeo*; Kato, Masato; Nelson, A.*; McClellan, K.*
Transactions of the American Nuclear Society, 113(1), p.617 - 618, 2015/10
Cerium dioxide has been used as a surrogate material for plutonium dioxide. Dorr et al reported the use of hyper-stoichiometric conditions causes the start of shrinkage of (U,Ce)O at low temperature compared with the sintering in reducing atmosphere. However, the precise stoichiometry of the samples investigated was not controlled or otherwise monitored, preventing any quantitative conclusions regarding the similarities or differences between (U,Ce)O
at low temperature compared with the sintering in reducing atmosphere. However, the precise stoichiometry of the samples investigated was not controlled or otherwise monitored, preventing any quantitative conclusions regarding the similarities or differences between (U,Ce)O and (U,Pu)O
and (U,Pu)O . The motivation for the present work is therefore to compare the sintering behavior of MOX and the (U,Ce)O
. The motivation for the present work is therefore to compare the sintering behavior of MOX and the (U,Ce)O MOX surrogates under controlled atmospheres to assess the role of oxygen defects on densification in both systems.
MOX surrogates under controlled atmospheres to assess the role of oxygen defects on densification in both systems.
 in comparison to UO
in comparison to UO and PuO
and PuO
Nelson, A. T.*; Rittman, D. R.*; White, J. T.*; Dunwoody, J. T.*; Kato, Masato; McClellan, K. J.*
Journal of the American Ceramic Society, 97(11), p.3652 - 3659, 2014/11
Times Cited Count:60 Percentile:93.26(Materials Science, Ceramics) -CeO
-CeO
Hirooka, Shun; Murakami, Tatsutoshi; Nelson, A. T.*; McClellan, K. J.*
INL/EXT-14-33515, p.34 - 36, 2014/10
Collaborative study has been done on the properties of nuclear materials between the DOE and Japan and the oxygen potential of (U,Ce)O was measured in this year. Experimental measurements of the oxygen potential were conducted on Ce=20% and 30% composition rates simulating Pu content in advanced MOX fuel in JAEA by gas equilibrium method where oxygen partial pressure in the atmosphere was controlled with mixing dry/wet Ar/H
was measured in this year. Experimental measurements of the oxygen potential were conducted on Ce=20% and 30% composition rates simulating Pu content in advanced MOX fuel in JAEA by gas equilibrium method where oxygen partial pressure in the atmosphere was controlled with mixing dry/wet Ar/H gas. More than 100 data points were obtained in the O/M range of 1.945
gas. More than 100 data points were obtained in the O/M range of 1.945  2.000 at 1200
2.000 at 1200 C, 1400
C, 1400 C and 1600
C and 1600 C. The experimental results were analyzed by the defect analysis and analytical equations were obtained to calculate O/M as functions of temperature and oxygen potential. From the comparison with that of (U,Pu)O
C. The experimental results were analyzed by the defect analysis and analytical equations were obtained to calculate O/M as functions of temperature and oxygen potential. From the comparison with that of (U,Pu)O , applicability of the same defect chemistry and S-style curve are common. Also, it is revealed that (U,Ce)O
, applicability of the same defect chemistry and S-style curve are common. Also, it is revealed that (U,Ce)O requires evidently higher oxygen potential for the O/M.
requires evidently higher oxygen potential for the O/M.
Harada, Hideo; Shibata, Keiichi; Nishio, Katsuhisa; Igashira, Masayuki*; Plompen, A.*; Hambsch, F.-J.*; Schillebeeckx, P.*; Gunsing, F.*; Ledoux, X.*; Palmiotti, G.*; et al.
NEA/NSC/WPEC/DOC(2014)446, 111 Pages, 2014/02
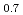 ,Pu
,Pu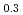 )O
)O in
in 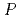
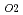 -controlled atmosphere
-controlled atmosphereKato, Masato; Murakami, Tatsutoshi; Sunaoshi, Takeo*; Nelson, A. T.*; McClellan, K.*
Proceedings of International Nuclear Fuel Cycle Conference; Nuclear Energy at a Crossroads (GLOBAL 2013) (CD-ROM), p.852 - 856, 2013/09
Uranium and plutonium mixed oxide (U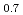 ,Pu
,Pu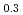 )O
)O which has been developed as a fast reactor fuel is nonstoichiometric compound. The stoichiometry significantly affects various properties. The relationship between oxygen potential and oxygen-to-metal (O/M) ratio has been investigated so far. The measurement results showed that it is essential to control oxygen partial pressure (
which has been developed as a fast reactor fuel is nonstoichiometric compound. The stoichiometry significantly affects various properties. The relationship between oxygen potential and oxygen-to-metal (O/M) ratio has been investigated so far. The measurement results showed that it is essential to control oxygen partial pressure (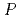
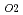 ) for holding constant O/M ratio in high temperature range. Therefore, properties of (U
) for holding constant O/M ratio in high temperature range. Therefore, properties of (U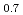 ,Pu
,Pu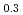 )O
)O were measured in
were measured in 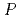
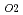 -controlled atmosphere in this work. In this work, properties of O/M change, sintering and thermal expansion were investigated in
-controlled atmosphere in this work. In this work, properties of O/M change, sintering and thermal expansion were investigated in 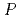
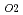 -controlled atmosphere.
-controlled atmosphere.
Hirooka, Shun; Kato, Masato; Tamura, Tetsuya*; Nelson, A. T.*; McClellan, K. J.*; Suzuki, Kiichi
Proceedings of International Conference on Fast Reactors and Related Fuel Cycles; Safe Technologies and Sustainable Scenarios (FR-13) (USB Flash Drive), 8 Pages, 2013/03
As research and development activities for MOX fuel pellet production, oxidation and reduction behaviors of MOX powders were investigated by thermogravimetry and X-ray diffraction measurements. It was observed that the oxidation limit decreased with oxidizing temperature and Pu content. The MOX powders showed a two-step oxidation and kinetic stability under non-stoichiometry. The oxidation rates were evaluated from the isothermal oxidation tests. It was found that the reduction temperature of M O
O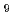 + M
+ M O
O was higher than that of M
was higher than that of M O
O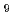 . This indicated that the reduction of M
. This indicated that the reduction of M O
O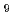 was prevented by the existence of M
was prevented by the existence of M O
O . Activation energy of the reduction was derived from the non-isothermal reduction tests. The data are expected to contribute to establishing a control technique for O/M ratio during MOX powder storage and pellet production.
. Activation energy of the reduction was derived from the non-isothermal reduction tests. The data are expected to contribute to establishing a control technique for O/M ratio during MOX powder storage and pellet production.
 Ca +
Ca +  Cm
Cm 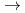
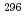 116
116 studied at the GSI-SHIP
studied at the GSI-SHIPHofmann, S.*; Heinz, S.*; Mann, R.*; Maurer, J.*; Khuyagbaatar, J.*; Ackermann, D.*; Antalic, S.*; Barth, B.*; Block, M.*; Burkhard, H. G.*; et al.
European Physical Journal A, 48(5), p.62_1 - 62_23, 2012/05
Times Cited Count:167 Percentile:98.87(Physics, Nuclear) in air
in airSuzuki, Kiichi; Nelson, A. T.*; Sunaoshi, Takeo*; McClellan, K.*; Kato, Masato
no journal, ,
In a reactor core or a spent fuel pool loss of coolant accident, fuel cladding may be breached and cause rapid oxidation of UO pellets. Oxidation of UO
pellets. Oxidation of UO results in pulverization of the pellet and significant evaporation of UO
results in pulverization of the pellet and significant evaporation of UO , possibly leading to spread of nuclear material to the environment. Therefore, understanding the oxidation behavior of UO
, possibly leading to spread of nuclear material to the environment. Therefore, understanding the oxidation behavior of UO is an important factor. In this study, the oxidation behavior of UO
is an important factor. In this study, the oxidation behavior of UO pellets and powders in air was investigated over a wide temperature range. Isothermal oxidations were carried out from 673 to 1923 K. Oxidative pulverization was observed below 1073 K. The weight gain rate at 773 K was larger than that at 873 K, suggesting the oxidation mechanism might change in this regime. Rapid weight loss was observed above 1723 K. The vapor pressure of each phase in the U-O system was calculated, indicating that volatilization of UO
pellets and powders in air was investigated over a wide temperature range. Isothermal oxidations were carried out from 673 to 1923 K. Oxidative pulverization was observed below 1073 K. The weight gain rate at 773 K was larger than that at 873 K, suggesting the oxidation mechanism might change in this regime. Rapid weight loss was observed above 1723 K. The vapor pressure of each phase in the U-O system was calculated, indicating that volatilization of UO was likely responsible for the measured weight loss. The evaporation rate of UO
was likely responsible for the measured weight loss. The evaporation rate of UO for a urania pellet was then evaluated.
for a urania pellet was then evaluated.
 powder in water vapor
powder in water vaporSuzuki, Kiichi; Kato, Masato; Nelson, A.*; McClellan, K.*
no journal, ,
no abstracts in English
 in oxygen partial pressure controlled atmosphere
in oxygen partial pressure controlled atmosphereKato, Masato; Ikusawa, Yoshihisa; Sunaoshi, Takeo*; McClellan, K.*; Nelson, A.*
no journal, ,
Thermal expansion of MOX was measured as a function of O/M ratio. The measurements of the thermal expansion and O/M were carried out with dilatometer and thermo gravimeter, respectively, in cooling process of 4 K/min. Oxygen potential of MOX has been measured, and the correlation was derived to represent the relationship between oxygen potential and O/M ratio. The P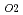 in the atmosphere was controlled according to the previous data which were controlled by adjusting PH
in the atmosphere was controlled according to the previous data which were controlled by adjusting PH /PH
/PH O ratio. It was confirmed to hold constant O/M ratio during the measurements by thermo-gravimetry. Thermal expansion of (U
O ratio. It was confirmed to hold constant O/M ratio during the measurements by thermo-gravimetry. Thermal expansion of (U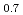 Pu
Pu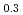 )O
)O increased with increasing the deviation x. The correlation to represent the thermal expansion was derived with Pu content and O/M ratio. The thermal expansion coefficients are consistent with those of PuO
increased with increasing the deviation x. The correlation to represent the thermal expansion was derived with Pu content and O/M ratio. The thermal expansion coefficients are consistent with those of PuO .
.

Murakami, Tatsutoshi; Kato, Masato; Nelson, A.*; McClellan, K.*
no journal, ,
no abstracts in English
Hirooka, Shun; Murakami, Tatsutoshi; Kato, Masato; Suzuki, Kiichi; Nelson, A.*; White, J.*; McClellan, K.*
no journal, ,
no abstracts in English
 thermal conductivity
thermal conductivityHirooka, Shun; Kato, Masato; Nelson, A.*; McClellan, K.*; White, J.*
no journal, ,
Thermal diffusivity of (U,Ce)O was measured as a function of O/M, and the thermal conductivity was calculated. Thermal conductivity of (U,Ce)O
was measured as a function of O/M, and the thermal conductivity was calculated. Thermal conductivity of (U,Ce)O decreased more sharply with increasing temperature compared with that of (U,Pu)O
decreased more sharply with increasing temperature compared with that of (U,Pu)O . Also, thermal conductivity of (U,Ce)O
. Also, thermal conductivity of (U,Ce)O showed the highest peak at O/M of 2 and decreased as the O/M is apart from 2 on both hypo-stoichiometric and hyper-stoichimetric regions.
showed the highest peak at O/M of 2 and decreased as the O/M is apart from 2 on both hypo-stoichiometric and hyper-stoichimetric regions.
White, J.*; Hirooka, Shun; Murakami, Tatsutoshi; Nelson, A.*; McClellan, K.*; Kato, Masato
no journal, ,
Use of CeO as a surrogate to better understand the behavior of MOX fuels requires a fundamental investigation of the thermophysical properties to assess the appropriate use. The challenge in performing thermophysical property measurements on oxygen non-toichiometric MOX is in handling the kinetics at elevated temperatures and also in determining a reference state from which to measure the oxygen:metal ratio (O:M). This study integrated the use of identical gas handling systems installed on a thermogravimetric analyzer and the property measurement system (differential scanning calorimeter and laser flash analyzer) to control the partial pressure of oxygen, PO
as a surrogate to better understand the behavior of MOX fuels requires a fundamental investigation of the thermophysical properties to assess the appropriate use. The challenge in performing thermophysical property measurements on oxygen non-toichiometric MOX is in handling the kinetics at elevated temperatures and also in determining a reference state from which to measure the oxygen:metal ratio (O:M). This study integrated the use of identical gas handling systems installed on a thermogravimetric analyzer and the property measurement system (differential scanning calorimeter and laser flash analyzer) to control the partial pressure of oxygen, PO , between the system and maintain stoichiometry from ambient temperature to 1200
, between the system and maintain stoichiometry from ambient temperature to 1200 C.
C.
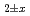
Suzuki, Kiichi; Murakami, Tatsutoshi; Hirooka, Shun; Nelson, A.*; McClellan, K.*; Kato, Masato
no journal, ,
The relationship between equilibrium oxygen partial pressure, 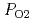 , and deviation from stoichiometric composition, x, was evaluated using a Kroger-Vink diagram. It was found that x was proportional to
, and deviation from stoichiometric composition, x, was evaluated using a Kroger-Vink diagram. It was found that x was proportional to 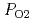
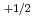 and
and 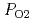
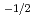 near stoichiometry for all compositions. This suggests that intrinsic ionization is dominant near stoichiometry. Also, in the reducing region, x was proportional to
near stoichiometry for all compositions. This suggests that intrinsic ionization is dominant near stoichiometry. Also, in the reducing region, x was proportional to 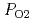
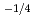 for 20 and 30% of Ce, and was proportional to
for 20 and 30% of Ce, and was proportional to 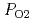
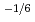 for 5% of Ce. Defect reactions and defect concentrations were evaluated by the relationship between x and
for 5% of Ce. Defect reactions and defect concentrations were evaluated by the relationship between x and 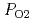
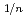 . Then, the oxygen potential expression was derived using the defect concentrations as functions of O/M ratio, temperature and Ce content. The calculated values reproduced experimental values well.
. Then, the oxygen potential expression was derived using the defect concentrations as functions of O/M ratio, temperature and Ce content. The calculated values reproduced experimental values well.
Hirooka, Shun; Nelson, A.*; White, J.*; Kato, Masato; McClellan, K.*
no journal, ,
Thermal diffusivity of (U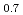 Ce
Ce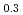 )O
)O and (U
and (U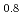 Ce
Ce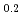 )O
)O was measured by laser flash method as a function of oxygen-to-metal ratio over the temperature range of 373 K to 1473 K and the thermal conductivity was evaluated. Only stoichiometric data of thermal conductivity of (U,Ce)O
was measured by laser flash method as a function of oxygen-to-metal ratio over the temperature range of 373 K to 1473 K and the thermal conductivity was evaluated. Only stoichiometric data of thermal conductivity of (U,Ce)O was reported and that was comparable with thermal conductivity obtained in this work. Thermal conductivity was highest at stoichiometry, and was not perfectly symmetric with respect to the stoichiometry but slightly lower for hyper-stoichiometric compositions. The thermal conductivity of (U,Ce)O
was reported and that was comparable with thermal conductivity obtained in this work. Thermal conductivity was highest at stoichiometry, and was not perfectly symmetric with respect to the stoichiometry but slightly lower for hyper-stoichiometric compositions. The thermal conductivity of (U,Ce)O showed the trend with temperature to be comparable to that of (U,Pu)O
showed the trend with temperature to be comparable to that of (U,Pu)O . However, the thermal resistivity did not follow the linear function of temperature, A+BT, which is an unique characteristics in (U,Ce)O
. However, the thermal resistivity did not follow the linear function of temperature, A+BT, which is an unique characteristics in (U,Ce)O .
.

Nakamichi, Shinya; Hirooka, Shun; Kato, Masato; Sunaoshi, Takeo*; Nelson, A.*; McClellan, K.*
no journal, ,
Sintering behavior of (U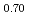 Ce
Ce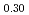 )O
)O was estimated and compared with that of MOX. Sintering of (U
was estimated and compared with that of MOX. Sintering of (U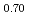 Ce
Ce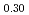 )O
)O in CO
in CO was not accelerated in contrast with that of MOX.
was not accelerated in contrast with that of MOX.