Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
糸井 弘行*; Ninomiya, Takeru*; 長谷川 英之*; Maki, Shintaro*; Sakakibara, Akihiro*; 鈴木 隆太郎*; 笠井 湧斗*; 岩田 博之*; 松村 大樹; 大和田 真生*; et al.
Journal of Physical Chemistry C, 124(28), p.15205 - 15215, 2020/07
被引用回数:8 パーセンタイル:40.15(Chemistry, Physical)We demonstrate reversible charge/discharge in ruthenocene, RuCp (Cp =
(Cp = 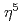 -C
-C H
H ), using activated carbon (AC) as a support. Upon subsequent electrochemical oxidation using an aqueous H
), using activated carbon (AC) as a support. Upon subsequent electrochemical oxidation using an aqueous H SO
SO electrolyte, the clusters are disassembled and the RuCp
electrolyte, the clusters are disassembled and the RuCp molecules are finely dispersed in the micropores. The resulting RuCp
molecules are finely dispersed in the micropores. The resulting RuCp has a large contact area with conductive carbon surfaces, thereby realizing rapid charge transfer at the contact interface. Consequently, rapid charge storage occurs via the reversible redox reaction of the supported RuCp
has a large contact area with conductive carbon surfaces, thereby realizing rapid charge transfer at the contact interface. Consequently, rapid charge storage occurs via the reversible redox reaction of the supported RuCp in aqueous H
in aqueous H SO
SO without dimerization or disproportionation reactions, which is confirmed by X-ray absorption spectroscopy. Since hybridization can produce different properties of the host and guest materials, their infinite combinations would have the possibility to yield properties far surpassing those of existing materials.
without dimerization or disproportionation reactions, which is confirmed by X-ray absorption spectroscopy. Since hybridization can produce different properties of the host and guest materials, their infinite combinations would have the possibility to yield properties far surpassing those of existing materials.
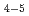 subnanoclusters and Pt single atoms over porous carbon supports and their structural analyses with X-ray absorption spectroscopy
subnanoclusters and Pt single atoms over porous carbon supports and their structural analyses with X-ray absorption spectroscopy糸井 弘行*; 西原 洋知*; 小林 俊介*; Ittisanronnachai, S.*; 石井 孝文*; Berenguer, R.*; 伊藤 仁*; 松村 大樹; 京谷 隆*
Journal of Physical Chemistry C, 121(14), p.7892 - 7902, 2017/04
被引用回数:31 パーセンタイル:71.76(Chemistry, Physical)We demonstrate the fine dispersion of Pt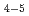 subnanoclusters or Pt single atoms on two types of porous carbon supports, Ketjen black (KB) and zeolite-templated carbon (ZTC). For the fine dispersion of Pt subnanoclusters, simply filling an organoplatinum complex, (COD)PtMe
subnanoclusters or Pt single atoms on two types of porous carbon supports, Ketjen black (KB) and zeolite-templated carbon (ZTC). For the fine dispersion of Pt subnanoclusters, simply filling an organoplatinum complex, (COD)PtMe , as a Pt precursor into the micropores is found to be crucial to prevent agglomeration or sintering of Pt subnanoclusters. Moreover, it is possible to disperse Pt single atoms on ZTC, simply by decreasing the Pt loading amount down to ca. 0.9 wt%, owing to some stabilization effect by oxygen-containing functional groups. Extended X-ray absorption fine structure (EXAFS) analysis for Pt subnanoclusters reveals that the Pt-Pt bond is contracting to a large extent (
, as a Pt precursor into the micropores is found to be crucial to prevent agglomeration or sintering of Pt subnanoclusters. Moreover, it is possible to disperse Pt single atoms on ZTC, simply by decreasing the Pt loading amount down to ca. 0.9 wt%, owing to some stabilization effect by oxygen-containing functional groups. Extended X-ray absorption fine structure (EXAFS) analysis for Pt subnanoclusters reveals that the Pt-Pt bond is contracting to a large extent ( 2.8%) relative to balk values, and upon hydrogen chemisorption, the bond elongates more than the reported values for the 1.0 nm sized Pt nanoparticles.
2.8%) relative to balk values, and upon hydrogen chemisorption, the bond elongates more than the reported values for the 1.0 nm sized Pt nanoparticles.