Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Reinecke, E.-A.*; Takenaka, Keisuke*; Ono, Hitomi*; Kita, Tomoaki*; Taniguchi, Masashi*; Nishihata, Yasuo; Hino, Ryutaro; Tanaka, Hirohisa*
International Journal of Hydrogen Energy, 46(23), p.12511 - 12521, 2021/03
Times Cited Count:5 Percentile:26.59(Chemistry, Physical)The safe decommissioning as well as decontamination of the radioactive waste resulting from the nuclear accident in Fukushima Daiichi represents a huge task for the next decade. At present, research and development on long-term safe storage containers has become an urgent task with international cooperation in Japan. One challenge is the generation of hydrogen and oxygen in significant amounts by means of radiolysis inside the containers, as the nuclear waste contains a large portion of sea water. The generation of radiolysis gases may lead to a significant pressure build-up inside the containers and to the formation of flammable gases with the risk of ignition and the loss of integrity. In the framework of the project "R&D on technology for reducing concentration of flammable gases generated in long-term waste storage containers" funded by the Japanese Ministry of Education, Culture, Sports, Science and Technology of Japan (MEXT), the potential application of catalytic recombiner devices inside the storage containers is investigated. In this context, a suitable catalyst based on the so-called intelligent automotive catalyst for use in a recombiner is under consideration. The catalyst is originally developed and mass-produced for automotive exhaust gas purification, and is characterized by having a self-healing function of precious metals (Pd, Pt and Rh) dissolved as a solid solution in the perovskite type oxides. The basic features of this catalyst have been tested in an experimental program. The test series in the REKO-4 facility has revealed the basic characteristics of the catalyst required for designing the recombiner system.
Ono, Hitomi*; Takenaka, Keisuke*; Kita, Tomoaki*; Taniguchi, Masashi*; Matsumura, Daiju; Nishihata, Yasuo; Hino, Ryutaro; Reinecke, E.-A.*; Takase, Kazuyuki*; Tanaka, Hirohisa*
E-Journal of Advanced Maintenance (Internet), 11(1), p.40 - 45, 2019/05
Kishi, Hirofumi*; Sakamoto, Tomokazu*; Asazawa, Koichiro*; Yamaguchi, Susumu*; Kato, Takeshi*; Zulevi, B.*; Serov, A.*; Artyushkova, K.*; Atanassov, P.*; Matsumura, Daiju; et al.
Nanomaterials (Internet), 8(12), p.965_1 - 965_13, 2018/12
Times Cited Count:11 Percentile:48.30(Chemistry, Multidisciplinary)Kim, J.*; Yamanaka, Satoru*; Nakajima, Akira*; Kato, Takanori*; Kim, Y.*; Fukuda, Tatsuo; Yoshii, Kenji; Nishihata, Yasuo; Baba, Masaaki*; Takeda, Masatoshi*; et al.
Advanced Sustainable Systems (Internet), 2(11), p.1800067_1 - 1800067_8, 2018/11
Times Cited Count:7 Percentile:27.19(Green & Sustainable Science & Technology)Moro, Takuya*; Kim, J.*; Yamanaka, Satoru*; Murayama, Ichiro*; Kato, Takanori*; Nakayama, Tadachika*; Takeda, Masatoshi*; Yamada, Noboru*; Nishihata, Yasuo; Fukuda, Tatsuo; et al.
Journal of Alloys and Compounds, 768, p.22 - 27, 2018/11
Times Cited Count:17 Percentile:65.14(Chemistry, Physical)Yoshigoe, Akitaka; Shiwaku, Hideaki; Kobayashi, Toru; Shimoyama, Iwao; Matsumura, Daiju; Tsuji, Takuya; Nishihata, Yasuo; Kogure, Toshihiro*; Okochi, Takuo*; Yasui, Akira*; et al.
Applied Physics Letters, 112(2), p.021603_1 - 021603_5, 2018/01
Times Cited Count:6 Percentile:29.42(Physics, Applied)A synchrotron radiation photoemission electron microscope (SR-PEEM) was applied to demonstrate pinpoint analysis of micrometer-sized weathered biotite clay particles with artificially adsorbed cesium (Cs) atoms. Despite the insulating properties of the clay, we observed the spatial distributions of constituent elements (Si, Al, Cs, Mg, Fe) without charging issues. We found that Cs atoms were likely to be adsorbed evenly over the entire particle. Spatially-resolved X-ray absorption spectra (XAS) of the Cs M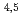 -edge region showed Cs to be present in a monocation state (Cs
-edge region showed Cs to be present in a monocation state (Cs ). Further pinpoint XAS measurements were also performed at the Fe L
). Further pinpoint XAS measurements were also performed at the Fe L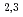 -edge to determine the chemical valence of the Fe atoms. Our results demonstrate the utility of SR-PEEM as a tool for spatially-resolved chemical analyses of various environmental substances, which is not limited by the poor conductivity of samples.
-edge to determine the chemical valence of the Fe atoms. Our results demonstrate the utility of SR-PEEM as a tool for spatially-resolved chemical analyses of various environmental substances, which is not limited by the poor conductivity of samples.
Kim, J.*; Yamanaka, Satoru*; Nakajima, Akira*; Kato, Takanori*; Kim, Y.*; Fukuda, Tatsuo; Yoshii, Kenji; Nishihata, Yasuo; Baba, Masaaki*; Takeda, Masatoshi*; et al.
Ferroelectrics, 512(1), p.92 - 99, 2017/08
Times Cited Count:14 Percentile:55.47(Materials Science, Multidisciplinary)Yamanaka, Satoru*; Kim, J.*; Nakajima, Akira*; Kato, Takanori*; Kim, Y.*; Fukuda, Tatsuo; Yoshii, Kenji; Nishihata, Yasuo; Baba, Masaaki*; Yamada, Noboru*; et al.
Advanced Sustainable Systems (Internet), 1(3-4), p.1600020_1 - 1600020_6, 2017/04
no abstracts in English
 X-ray absorption spectroscopy study on water formation reaction of palladium metal nanoparticle catalysts
X-ray absorption spectroscopy study on water formation reaction of palladium metal nanoparticle catalystsMatsumura, Daiju; Taniguchi, Masashi*; Tanaka, Hirohisa*; Nishihata, Yasuo
International Journal of Hydrogen Energy, 42(11), p.7749 - 7754, 2017/03
Times Cited Count:5 Percentile:13.82(Chemistry, Physical)Tamura, Kazuhisa; Nishihata, Yasuo
Journal of Physical Chemistry C, 120(29), p.15691 - 15697, 2016/07
Times Cited Count:9 Percentile:31.44(Chemistry, Physical)The behavior of halide ions on the Au(111) electrode surface in two ionic liquids (ILs) was investigated by monitoring the structure of the electrode surface. The potential dependences of the X-ray diffraction intensity, which originate from the Au(111)-(1 1) structure and the surface normal structure, were measured simultaneously with cyclic voltammograms. The results revealed that halide ions are co-adsorbed with IL molecules on the electrode surface and increase the mobility of surface atoms. This suggests that the interaction between halide ions and surface Au atoms is weaker than that between IL molecules and surface Au atoms; that is, the surface properties are mainly governed by adsorbed IL molecules. Furthermore, a comparison of the two ILs revealed that the effect of halide ions on the structure of the Au(111) electrode surface depends on the strength of the interaction between IL molecules and surface Au atoms.
1) structure and the surface normal structure, were measured simultaneously with cyclic voltammograms. The results revealed that halide ions are co-adsorbed with IL molecules on the electrode surface and increase the mobility of surface atoms. This suggests that the interaction between halide ions and surface Au atoms is weaker than that between IL molecules and surface Au atoms; that is, the surface properties are mainly governed by adsorbed IL molecules. Furthermore, a comparison of the two ILs revealed that the effect of halide ions on the structure of the Au(111) electrode surface depends on the strength of the interaction between IL molecules and surface Au atoms.
 O
O studied by time-resolved XAFS using dispersive optics
studied by time-resolved XAFS using dispersive opticsMatsumura, Daiju; Okajima, Yuka*; Nishihata, Yasuo
Journal of Physics; Conference Series, 712(1), p.012042_1 - 012042_4, 2016/06
Times Cited Count:0 Percentile:0.03(Physics, Applied)Sakamoto, Tomokazu*; Kishi, Hirofumi*; Yamaguchi, Susumu*; Tanaka, Hirohisa*; Matsumura, Daiju; Tamura, Kazuhisa; Nishihata, Yasuo
Hyomen Kagaku, 37(2), p.78 - 83, 2016/02
We have developed direct liquid fuel anion exchange membrane fuel cell vehicles to deal with the global warming. Non-platinum group metals (PGM) catalyst has been researched to apply for both anode and cathode electrodes. A test driving was carried out for the fuel cell vehicle equipped with no precious metals as catalysts at SPring-8 in 2013. Here we introduce our results of advanced analysis for reaction mechanism and active site of non-PGM catalyst using synchrotron radiation X-rays at SPring-8.
Torigoe, Shuhei*; Ishimoto, Yutaro*; Aoishi, Yuhei*; Murakawa, Hiroshi*; Matsumura, Daiju; Yoshii, Kenji; Yoneda, Yasuhiro; Nishihata, Yasuo; Kodama, Katsuaki; Tomiyasu, Keisuke*; et al.
Physical Review B, 93(8), p.085109_1 - 085109_5, 2016/02
Times Cited Count:5 Percentile:25.63(Materials Science, Multidisciplinary)Matsumura, Daiju; Nishihata, Yasuo; Okajima, Yuka*
e-Journal of Surface Science and Nanotechnology (Internet), 14, p.48 - 52, 2016/00
Kamiji, Yu; Taniguchi, Masashi*; Nishihata, Yasuo; Nagaishi, Ryuji; Tanaka, Hirohisa*; Hirata, Shingo*; Hara, Mikiya; Hino, Ryutaro
E-Journal of Advanced Maintenance (Internet), 7(1), p.84 - 89, 2015/05
For hydrogen mitigation, a new type passive autocatalytic recombiner is under developing. This new recombiner has been developed from automotive monolithic catalyst in order to reduce weight and to improve hydrogen treating capacity, environmental resistance and product quality. In this study, activation energy of hydrogen-oxygen recombination reaction was examined to clarify the basic characteristics of the catalyst. In addition, the degradation of the catalyst by  -ray irradiation simulating the environmental condition in nuclear power plants was also examined. As a result, the activation energy was experimentally estimated at 5.75 kJ/mol. Besides, no significant differences were observed in compositional distribution from the EPMA results. On the other hand, specific surface area of the catalyst and surface area of the precious metals were increased. Moreover, catalyst performance test showed that
-ray irradiation simulating the environmental condition in nuclear power plants was also examined. As a result, the activation energy was experimentally estimated at 5.75 kJ/mol. Besides, no significant differences were observed in compositional distribution from the EPMA results. On the other hand, specific surface area of the catalyst and surface area of the precious metals were increased. Moreover, catalyst performance test showed that  -ray irradiation up to 1.0 MGy can increase activity of catalyst.
-ray irradiation up to 1.0 MGy can increase activity of catalyst.
Sakamoto, Tomokazu*; Matsumura, Daiju; Asazawa, Koichiro*; Martinez, U.*; Serov, A.*; Artyushkova, K.*; Atanassov, P.*; Tamura, Kazuhisa; Nishihata, Yasuo; Tanaka, Hirohisa*
Electrochimica Acta, 163, p.116 - 122, 2015/05
Times Cited Count:57 Percentile:83.30(Electrochemistry)Kamiji, Yu; Matsumura, Daiju; Taniguchi, Masashi*; Nishihata, Yasuo; Tanaka, Hirohisa*; Hirata, Shingo*; Hara, Mikiya; Hino, Ryutaro
Proceedings of 23rd International Conference on Nuclear Engineering (ICONE-23) (DVD-ROM), 4 Pages, 2015/05
In a severe accident at a nuclear power plant, a large amount of hydrogen can be released to primary containment vessel or reactor building. Passive autocatalytic recombiner (PAR) is one of the most effective systems for hydrogen mitigation and safety accident management. The new type PAR is under developing to improve conventional PARs, especially its size and weight. In this study, the influence of steam coexistence for the automotive catalyst activity was experimentally examined. These results show that the steam slightly affects the reaction start up and catalyst activity.
Jarrige, I.*; Ishii, Kenji; Matsumura, Daiju; Nishihata, Yasuo; Yoshida, Masahiro*; Kishi, Hirofumi*; Taniguchi, Masashi*; Uenishi, Mari*; Tanaka, Hirohisa*; Kasai, Hideaki*; et al.
ACS Catalysis, 5(2), p.1112 - 1118, 2015/02
Times Cited Count:18 Percentile:43.51(Chemistry, Physical)Matsumura, Daiju; Kobayashi, Toru; Miyazaki, Yuji; Okajima, Yuka*; Nishihata, Yasuo; Yaita, Tsuyoshi
Clay Science, 18(4), p.99 - 105, 2014/12
Tsuji, Takuya; Matsumura, Daiju; Kobayashi, Toru; Suzuki, Shinichi; Yoshii, Kenji; Nishihata, Yasuo; Yaita, Tsuyoshi
Clay Science, 18(4), p.93 - 97, 2014/12