Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Suzuki, Tomoya; Takao, Koichiro*; Kawasaki, Takeshi*; Harada, Masayuki*; Nogami, Masanobu*; Ikeda, Yasuhisa*
Polyhedron, 96, p.102 - 106, 2015/08
Times Cited Count:8 Percentile:52.61(Chemistry, Inorganic & Nuclear)We have determined crystal structures of UO (NO
(NO )
) (
(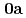 )
) (
(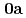 : 2-imidazolidone), UO
: 2-imidazolidone), UO (NO
(NO )
) (
(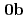 )
) (
(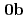 : tetrahydro-2-pyrimidone) and UO
: tetrahydro-2-pyrimidone) and UO (NO
(NO )
) (
(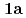 )
) (
(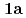 : 1-methyl-2-imidazolidone) by using single crystal X-ray analysis, and examined correlations between melting points (mps) and intermolecular hydrogen bonds (HBs) of UO
: 1-methyl-2-imidazolidone) by using single crystal X-ray analysis, and examined correlations between melting points (mps) and intermolecular hydrogen bonds (HBs) of UO (NO
(NO )
) (CU)
(CU) (CU: cyclic urea derivatives) and UO
(CU: cyclic urea derivatives) and UO (NO
(NO )
) (NRP)
(NRP) (NRP: pyrrolidone derivative).
(NRP: pyrrolidone derivative).
 -alkylated 2-pyrrolidone derivatives
-alkylated 2-pyrrolidone derivativesTakao, Koichiro*; Kawata, Yoshihisa*; Nogami, Masanobu*; Harada, Masayuki*; Morita, Yasuji; Nishimura, Kenji*; Ikeda, Yasuhisa*
Journal of Nuclear Science and Technology, 52(2), p.294 - 298, 2015/02
Times Cited Count:2 Percentile:16.17(Nuclear Science & Technology)Yields of precipitated UO (NO
(NO )
) (NRP)
(NRP) (NRP =
(NRP =  -alkylated 2-pyrrolidone) were precisely determined by considering reduction of the solution volume through the precipitation, which can be estimated from difference in acid concentrations of the liquid phases before and after the precipitation. The studied NRPs were
-alkylated 2-pyrrolidone) were precisely determined by considering reduction of the solution volume through the precipitation, which can be estimated from difference in acid concentrations of the liquid phases before and after the precipitation. The studied NRPs were  -
- -butyl (NBP) and
-butyl (NBP) and  -
- -propyl (NProP) derivatives. In both systems, the precipitation yields precisely determined were always higher than those simply calculated from the ratio of uranium concentrations before and after the precipitation. However, the differences between them are in the range of 0.6% - 2.6%. If such a difference is practically negligible, the volume reduction through the precipitation does not have to be taken into account for simplicity of the analytical manipulation.
-propyl (NProP) derivatives. In both systems, the precipitation yields precisely determined were always higher than those simply calculated from the ratio of uranium concentrations before and after the precipitation. However, the differences between them are in the range of 0.6% - 2.6%. If such a difference is practically negligible, the volume reduction through the precipitation does not have to be taken into account for simplicity of the analytical manipulation.
 -ray irradiation in HNO
-ray irradiation in HNO media
mediaNogami, Masanobu*; Sugiyama, Yuichi*; Kawasaki, Takeshi*; Harada, Masayuki*; Kawata, Yoshihisa*; Morita, Yasuji; Kikuchi, Toshiaki*; Ikeda, Yasuhisa*
Journal of Radioanalytical and Nuclear Chemistry, 296(1), p.423 - 427, 2013/04
Times Cited Count:6 Percentile:41.67(Chemistry, Analytical)Stability of polyvinylpolypyrrolidone (PVPP), a resin with adsorption selectivity to U(VI) in nitric acid media, against  -ray irradiation has been examined using HNO
-ray irradiation has been examined using HNO solutions of various concentrations. As the result, no clear decrease in the capacity was observed for any samples. Or rather, it was found that the capacity increased by approximately 50% for the PVPP slurry irradiated in 6 M HNO
solutions of various concentrations. As the result, no clear decrease in the capacity was observed for any samples. Or rather, it was found that the capacity increased by approximately 50% for the PVPP slurry irradiated in 6 M HNO . The infrared spectroscopic study indicates that PVPP degrades by
. The infrared spectroscopic study indicates that PVPP degrades by  -ray irradiation in HNO
-ray irradiation in HNO from the cleavage of the pyrrolidone ring by the addition of oxygen atom originating from HNO
from the cleavage of the pyrrolidone ring by the addition of oxygen atom originating from HNO , followed by the formation of chain monoamides with multiple coordinative atoms by the continuous addition of oxygen, finally leading to the generation of primary-amine type anion exchange resin. It is also indicated that all generated functional groups possess adsorptivity to U(VI) in 3 M HNO
, followed by the formation of chain monoamides with multiple coordinative atoms by the continuous addition of oxygen, finally leading to the generation of primary-amine type anion exchange resin. It is also indicated that all generated functional groups possess adsorptivity to U(VI) in 3 M HNO .
.
 -ray irradiation
-ray irradiationNogami, Masanobu*; Sugiyama, Yuichi*; Kawasaki, Takeshi*; Harada, Masayuki*; Kawata, Yoshihisa*; Morita, Yasuji; Kikuchi, Toshiaki*; Ikeda, Yasuhisa*
Science China; Chemistry, 55(9), p.1739 - 1745, 2012/09
Times Cited Count:4 Percentile:19.06(Chemistry, Multidisciplinary)Stability of N-alkylated pyrrolidone derivatives (NRPs) against radiation was examined by irradiation tests with 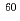 Co
Co  -ray. We have been developed a novel reprocessing system using NRPs which have precipitation ability to haxa- and tetravalent actinides in nitric acid media. Degradation rates of NRPs are evaluated by irradiation in 3M nitric acid solutions and mechanism of degradation are discussed in the present paper.
-ray. We have been developed a novel reprocessing system using NRPs which have precipitation ability to haxa- and tetravalent actinides in nitric acid media. Degradation rates of NRPs are evaluated by irradiation in 3M nitric acid solutions and mechanism of degradation are discussed in the present paper.
Ikeda, Yasuhisa*; Kawasaki, Takeshi*; Harada, Masayuki*; Nogami, Masanobu*; Kawata, Yoshihisa*; Kim, S.-Y.*; Morita, Yasuji; Chikazawa, Takahiro*; Someya, Hiroshi*; Kikuchi, Toshiaki*
Proceedings of International Conference on Toward and Over the Fukushima Daiichi Accident (GLOBAL 2011) (CD-ROM), 5 Pages, 2011/12
An advanced reprocessing system for spent FBR fuels based on two precipitation processes using pyrrolidone derivatives as precipitants has been developed. Experimental results of precipitation behavior of U, Pu and other elements, the heat- and radiation-resistance of precipitants, the thermal decomposition properties of precipitates showed that N-n-butyl-2-pyrrolidone and N-neopentyl-2-pyrrolidone are the appropriate precipitants for the first and second precipitation steps, respectively. From the engineering investigation, We confirmed that the precipitation and the filtration can be done efficiently using the engineering scale equipment and that the fuel pellets are directly prepared by the calcination of the precipitates. On the basis of these results, we evaluated that the proposed system is expected to be one of candidates of the future reprocessing systems for spent FBR fuels.
Nogami, Masanobu*; Harada, Masayuki*; Sugiyama, Yuichi*; Kawasaki, Takeshi*; Kawata, Yoshihisa*; Morita, Yasuji; Kikuchi, Toshiaki*; Ikeda, Yasuhisa*
Progress in Nuclear Energy, 53(7), p.948 - 951, 2011/09
Times Cited Count:4 Percentile:31.26(Nuclear Science & Technology)The precipitation ability of 1,3-dimethyl-2-imidazolidone (DMI) to U(VI) and U(IV) was examined using nitric acid solutions. While DMI precipitated U(VI) from 3 M nitric acid, no precipitate was observed in the solution containing 0.15 M U(IV) and 3 M nitric acid by adding DMI at the ratio of [DMI]/[U(IV)]=5. This indicates that the selectivity of DMI to U(VI) than U(IV). In spite of the excellent selectivity to U(VI), DMI has a disadvantage on the stability in nitric acid, because gradual acid hydrolysis of DMI is inevitable due to the nature of the chemical structure. Experiments on the stability of DMI in  -ray irradiation and heating in nitric acid solutions showed that the stability is strongly affected by the concentration of nitric acid and that DMI may be applicable in nitric acid solutions up to ca. 2 M.
-ray irradiation and heating in nitric acid solutions showed that the stability is strongly affected by the concentration of nitric acid and that DMI may be applicable in nitric acid solutions up to ca. 2 M.
Nogami, Masanobu*; Sugiyama, Yuichi*; Kawasaki, Takeshi*; Harada, Masayuki*; Morita, Yasuji; Kikuchi, Toshiaki*; Ikeda, Yasuhisa*
Journal of Radioanalytical and Nuclear Chemistry, 283(2), p.541 - 546, 2010/02
Times Cited Count:20 Percentile:77.17(Chemistry, Analytical)Adsorptivity of polyvinylpolypyrrolidone (PVPP) to various metal ions was examined as a part of the development of resins with selectivity to U(VI) in nitric acid media. It was found that PVPP has a strong adsorptivity to U(VI) in wide concentration range of nitric acid. The mechanism of U(VI) adsorption by PVPP was discussed by results of Scatchard plot analysis and infrared spectroscopic analysis. Furthermore, it was found that fission productions except for Re(VII) as the simulant of Tc(VII) and Pd(II) are not adsorbed on to PVPP and that Pd(II) and Re(VII) species are weakly adsorbed in the lower concentration ranges of nitric acid, where the adsorption rates of Pd(II) are extremely slower than those of U(VI). These results indicate that U(VI) can be separated from other metal ions by PVPP.
 -ray irradiation
-ray irradiationNogami, Masanobu*; Sugiyama, Yuichi*; Kawasaki, Takeshi*; Harada, Masayuki*; Kawata, Yoshihisa; Morita, Yasuji; Kikuchi, Toshiaki*; Ikeda, Yasuhisa*
JAEA-Review 2009-041, JAEA Takasaki Annual Report 2008, P. 25, 2009/12
As a part of the development of a novel reprocessing system for spent FBR fuels based on the precipitation method, influence of concentrations of HNO on the stability by
on the stability by  -ray irradiation was examined for
-ray irradiation was examined for  -
- -butyl-2-pyrrolidone (NBP), a candidate precipitant for the first precipitation step for selectively precipitating U(VI). The residual ratios of the samples for HNO
-butyl-2-pyrrolidone (NBP), a candidate precipitant for the first precipitation step for selectively precipitating U(VI). The residual ratios of the samples for HNO solutions up to 3 M were found to be decreased identically, where ca. 20% of NBP was degraded after the irradiation of 1 MGy. It was found that the degradation of the samples of 6 M HNO
solutions up to 3 M were found to be decreased identically, where ca. 20% of NBP was degraded after the irradiation of 1 MGy. It was found that the degradation of the samples of 6 M HNO is more distinguished, where ca. 30% was degraded after the irradiation of 0.1 MGy. As the result of the investigation of the degradation mechanism of NBP, it was revealed that the degradation started from the cleavage of the pyrrolidone ring of NBP by the addition of oxygen atom, followed by the formation of chain monoamides and C4 compounds by the continuous addition of oxygen, leading to the generation of oxalic acid.
is more distinguished, where ca. 30% was degraded after the irradiation of 0.1 MGy. As the result of the investigation of the degradation mechanism of NBP, it was revealed that the degradation started from the cleavage of the pyrrolidone ring of NBP by the addition of oxygen atom, followed by the formation of chain monoamides and C4 compounds by the continuous addition of oxygen, leading to the generation of oxalic acid.
Morita, Yasuji; Takao, Koichiro*; Kim, S.-Y.; Kawata, Yoshihisa; Harada, Masayuki*; Nogami, Masanobu*; Nishimura, Kenji*; Ikeda, Yasuhisa*
Journal of Nuclear Science and Technology, 46(12), p.1129 - 1136, 2009/12
Times Cited Count:18 Percentile:73.31(Nuclear Science & Technology)A reprocessing system for spent FBR fuels consisting of two precipitation processes has been proposed. In this system, first only U(IV) species are precipitated using pyrrolidone derivative with low hydrophobicity and donicity, and secondly residual U(VI) and Pu(IV, VI) are precipitated simultaneously using pyrrolidone derivative with high precipitation ability. In this study, we have examined precipitation behavior of U(VI), Pu(IV), and Pu(VI) species in nitric acid solutions by using  -
- -propyl-2-pyrrolidone (NProP),
-propyl-2-pyrrolidone (NProP),  -butyl-2-pyrrolidone (NBP),
-butyl-2-pyrrolidone (NBP),  -
-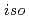 -butyl-2-pyrrolidone (NiBP), or
-butyl-2-pyrrolidone (NiBP), or  -cyclohexyl-2-pyrrolidone (NCP) to select the precipitants for the first precipitation process. As a result, NBP were found to be the most promising precipitant for the first precipitation process.
-cyclohexyl-2-pyrrolidone (NCP) to select the precipitants for the first precipitation process. As a result, NBP were found to be the most promising precipitant for the first precipitation process.
 -Alkyl-2-pyrrolidone derivatives (Alkyl =
-Alkyl-2-pyrrolidone derivatives (Alkyl =  -propyl,
-propyl,  -butyl,
-butyl, 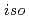 -butyl, and cyclohexyl)
-butyl, and cyclohexyl)Takao, Koichiro*; Noda, Kyoko*; Nogami, Masanobu*; Sugiyama, Yuichi*; Harada, Masayuki*; Morita, Yasuji; Nishimura, Kenji*; Ikeda, Yasuhisa*
Journal of Nuclear Science and Technology, 46(10), p.995 - 999, 2009/10
Times Cited Count:14 Percentile:65.50(Nuclear Science & Technology)We have been developing an advanced reprocessing system for spent FBR fuels based on precipitation method using pyrrolidone derivatives. In the present study, the solubility of UO (NO
(NO )
) (NRP)
(NRP) (NRP =
(NRP =  -alkyl-2-pyrrolidone, alkyl =
-alkyl-2-pyrrolidone, alkyl =  -propyl,
-propyl,  -butyl,
-butyl, 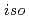 -butyl and cyclohexyl) in aqueous solutions with HNO
-butyl and cyclohexyl) in aqueous solutions with HNO has been examined. As a result, the solubility of each species of UO
has been examined. As a result, the solubility of each species of UO (NO
(NO )
) (NRP)
(NRP) generally decreases with increasing concentrations of HNO
generally decreases with increasing concentrations of HNO and NRP (
and NRP (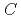 (HNO
(HNO ) and
) and 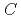 (NRP), respectively). The solubility of UO
(NRP), respectively). The solubility of UO (NO
(NO )
) (NRP)
(NRP) also depends on the type of NRP; a higher hydrophobicity of NRP generally leads to a lower solubility of UO
also depends on the type of NRP; a higher hydrophobicity of NRP generally leads to a lower solubility of UO (NO
(NO )
) (NRP)
(NRP) . The logarithms of effective solubility products (
. The logarithms of effective solubility products (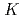
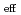 ) of UO
) of UO (NO
(NO )
) (NRP)
(NRP) at different
at different 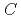 (HNO
(HNO ) values and 293 K were evaluated.
) values and 293 K were evaluated.
Ikeda, Yasuhisa*; Takao, Koichiro*; Harada, Masayuki*; Morita, Yasuji; Nogami, Masanobu*; Nishimura, Kenji*
Proceedings of International Conference on Advanced Nuclear Fuel Cycles and Systems (Global 2007) (CD-ROM), p.1503 - 1507, 2007/09
We have developed a reprocessing process for spent FBR fuels based on the precipitation method using pyrrolidone derivatives. In previous investigation, N-cyclohexyl-2-pyrrolidone (NCP) is used as a precipitant and a process consisting of selective U precipitation step and U-Pu co-precipitation step was developed. In the present study, in order to examine the applicability of precipitants with lower hydrophobicity than NCP to the selective U precipitation step, we have carried out precipitation experiments of U(VI) by N-butyl-2-pyrrolidone (NBP) and N-propyl-2-pyrrolidone (NProP) and measured decontamination factors of some fission products.
Morita, Yasuji; Kim, S.-Y.; Ikeda, Yasuhisa*; Nogami, Masanobu*; Nishimura, Kenji*
Proceedings of International Conference on Advanced Nuclear Fuel Cycles and Systems (Global 2007) (CD-ROM), p.1508 - 1512, 2007/09
We have been developing an advanced reprocessing system for spent FBR fuels based on precipitation method using pyrrolidone derivatives. In previous investigation, N-cyclohexyl-2-pyrrolidone (NCP) is used as a precipitant and a process consisting of selective U precipitation step and U-Pu co-precipitation step was developed. In order to make the process more effective and more economical, we are now studying precipitation of U and Pu with other pyrrolidone derivatives. In the present study, precipitation behavior of Pu was examined using N-butyl-2-pyrrolidone (NBP) and N-propyl-2-pyrrolidone (NProP), which have lower hydrophobicity than NCP. The experiments with Pu(IV) or Pu(VI) solutiona and U(VI)-Pu(IV) solutions showed that Pu is less precipitated with NBP or NProP than with NCP. From these results, it is expected that NBP and NProP can be used as precipitants for the selective U precipitation step and make the step more selective and effective.
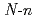 -butyl-2-pyrrolidone,
-butyl-2-pyrrolidone,  -cyclohexylmethyl-2-pyrrolidone, and 1,3-dimethyl-2-imidazolidone
-cyclohexylmethyl-2-pyrrolidone, and 1,3-dimethyl-2-imidazolidoneKoshino, Nobuyoshi*; Harada, Masayuki*; Nogami, Masanobu*; Morita, Yasuji; Kikuchi, Toshiaki*; Ikeda, Yasuhisa*
Inorganica Chimica Acta, 358(6), p.1857 - 1864, 2005/03
Times Cited Count:54 Percentile:87.98(Chemistry, Inorganic & Nuclear)Structural analyses of UO (NO
(NO )
) L
L [L=
[L=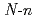 -butyl-2-pyrrolidone, N-cyclohexylmethyl-2-pyrrolidone, and 1,3-dimethyl-2-imidazolidone] have been carried out using X-ray diffraction method. These uranyl complexes were found to have a hexagonal bipyramidal structure. The bond distances of U=O and U-O (ligand), and bond angles of U-O-C(carbonyl) are determined. In uranyl nitrate complexes with cyclic amides such as 2-pyrrolidone, urea, and caprolactam derivatives, a linear correlation was found to hold between U-O (ligand) bond distances and U-O-C(carbonyl) bond angles. Vibrational frequencies of UO
-butyl-2-pyrrolidone, N-cyclohexylmethyl-2-pyrrolidone, and 1,3-dimethyl-2-imidazolidone] have been carried out using X-ray diffraction method. These uranyl complexes were found to have a hexagonal bipyramidal structure. The bond distances of U=O and U-O (ligand), and bond angles of U-O-C(carbonyl) are determined. In uranyl nitrate complexes with cyclic amides such as 2-pyrrolidone, urea, and caprolactam derivatives, a linear correlation was found to hold between U-O (ligand) bond distances and U-O-C(carbonyl) bond angles. Vibrational frequencies of UO (NO
(NO )
) L
L have also been measured by IR and Raman spectrophotometers. Using relationships between vibrational frequencies of O=U=O bonds and donor numbers (DNs) of ligands, donicities of N-substituted-2-pyrrolidones were determined.
have also been measured by IR and Raman spectrophotometers. Using relationships between vibrational frequencies of O=U=O bonds and donor numbers (DNs) of ligands, donicities of N-substituted-2-pyrrolidones were determined.
Harada, Masayuki*; Morita, Yasuji; Nogami, Masanobu*; Nishimura, Kenji*; Ikeda, Yasuhisa*
no journal, ,
In the present study, we have examined the precipitation ability of N-butyl-2-pyrrolidone (NBP) and N-propyl-2-pyrrolidone (NProP) as selective precipitants for uranyl ion, which have lower hydrophobicity than N-cycrohexyl-2-pyrrolidone (NCP) we had studied previously. As a result, we found that NBP and NProP can precipitate uranyl ions stoichiometrically regardless of the concentrations of uranyl ion and nitric acid, and the temperature of solutions. Furthermore, in the Pu concentration range of 0.04 to 0.06 M, Pu(IV) and Pu(VI) species were not precipitated by NProP, while Pu(IV) species are precipitated with addition of excess amount of NBP. From these results, it is expected that in the first precipitation process we can precipitate uranyl ions without coprecipitation of Pu(IV) species by using NBP or NProP.
Harada, Masayuki*; Ikeda, Yasuhisa*; Asakura, Toshihide; Morita, Yasuji; Nogami, Masanobu*; Nishimura, Kenji*
no journal, ,
Precipitation behavior of U(VI), Pu(IV) and Pu(VI) with pyrrolidone derivatives of N-butyl-2-pyrrolidone (NBP) and N-propyl-2-pyrrolidone (NProP) was examined to develop a simple and economical reprocessing process of spent nuclear fuel based only on precipitation method. These precipitants have lower donicity and hydrophobicity than N-cyclohexyl-2-pyrrolidone (NCP) which has already been examined for U(VI) precipitation. The addition of twice amount of the precipitant with molar ratio to U(VI) gave 70% precipitation of U(VI) from nitric acid solution of 2M U. Experiments on Pu precipitation behavior revealed that both NBP and NProP have lower ability to make precipitates of Pu(IV) and Pu(VI), which would contribute to the development of the efficient precipitation step only for U(VI).
Takao, Koichiro*; Noda, Kyoko*; Nogami, Masanobu*; Sugiyama, Yuichi*; Harada, Masayuki*; Ikeda, Yasuhisa*; Morita, Yasuji; Nishimura, Kenji*
no journal, ,
Precipitation behavior of U(VI) and some fission products (FP) with pyrrolidone derivatives of N-(1,2-dimethyl)propyl-2-pyrrolidone (NDMProP) and N-neopenthyl-2-pyrrolidone (NNpP) in the solutions of U-FP mixture has been examined in order to evaluate their applicability to the U-Pu co-precipitation process in the reprocessing based only on precipitation method. We have previously developed a process with N-cyclohexyl-2-pyrrolidone (NCP). It was found that U(VI) was precipitated in a high yield with any of the three precipitants and the order of the precipitation ability for U(VI) was NCP NNpP
NNpP NDMProP. Decontamination factors (DF) against FP except Zr(IV) and Mo(VI) in the U(VI) precipitation were over 100, and therefore NNpP and NDMProP can be used as an alternative precipitants to NCP.
NDMProP. Decontamination factors (DF) against FP except Zr(IV) and Mo(VI) in the U(VI) precipitation were over 100, and therefore NNpP and NDMProP can be used as an alternative precipitants to NCP.
Nogami, Masanobu*; Noda, Kyoko*; Takao, Koichiro*; Sugiyama, Yuichi*; Harada, Masayuki*; Ikeda, Yasuhisa*; Kawata, Yoshihisa; Morita, Yasuji; Nishimura, Kenji*
no journal, ,
We have been developing an advanced reprocessing system for spent FBR fuels based on precipitation method using pyrrolidone derivatives. The present study deals with durability of new precipitants with low and high hydrophobicity against  -irradiation and heating. Results showed that the precipitants with low hydrophobicity, N-n-butyl-2-pyrrolidone, N-propyl-2-pyrrolidone and N-iso-butyl-2-pyrrolidone, have enough durability against
-irradiation and heating. Results showed that the precipitants with low hydrophobicity, N-n-butyl-2-pyrrolidone, N-propyl-2-pyrrolidone and N-iso-butyl-2-pyrrolidone, have enough durability against  -irradiation. The precipitants with high hydrophobicity, N-cyclohexyl-2-pyrrolidone, N-(1,2-dimethyl)propyl-2-pyrrolidone and N-neopenthyl-2-pyrrolidone, also have enough durability but gave lower precipitation yield when they irradiated with higher dose rate. The precipitation ability of all the precipitants did not changed by the heating at 50
-irradiation. The precipitants with high hydrophobicity, N-cyclohexyl-2-pyrrolidone, N-(1,2-dimethyl)propyl-2-pyrrolidone and N-neopenthyl-2-pyrrolidone, also have enough durability but gave lower precipitation yield when they irradiated with higher dose rate. The precipitation ability of all the precipitants did not changed by the heating at 50 C for three days.
C for three days.
Harada, Masayuki*; Nogami, Masanobu*; Sugiyama, Yuichi*; Kawasaki, Takeshi*; Ikeda, Yasuhisa*; Kawata, Yoshihisa*; Morita, Yasuji; Kikuchi, Toshiaki*
no journal, ,
We have been developing an advanced reprocessing system for spent FBR fuels based on precipitation method using pyrrolidone derivatives. In the present study, the masking effect of some reagents was examined for the purpose of the reduction of Pu(IV) co-precipitation in the first selective U precipitation step using N-n-butyl-2-pyrrolidone (NBP) as a precipitant. Experiments with nitric acid solutions of U(VI)-Pu(IV) showed the masking effect of N-n-methyl-2-pyrrolidone (NMP) for Pu(IV).
Kawasaki, Takeshi*; Nogami, Masanobu*; Sugiyama, Yuichi*; Harada, Masayuki*; Ikeda, Yasuhisa*; Morita, Yasuji; Kikuchi, Toshiaki*
no journal, ,
We have been developing an advanced reprocessing system for spent FBR fuels based on precipitation method using pyrrolidone derivatives. In the present study, the recovery of precipitant from the precipitate through vaporization by heating was investigated using N-n-butyl-2-pyrrolidone (NBP) and N-n-neopentyl-2-pyrrolidone (NNpP) as precipitants. Experimental results showed that the most of the precipitants can be recovered without degradation and therefore the precipitants can be reused.
 -ray irradiation
-ray irradiationNogami, Masanobu*; Sugiyama, Yuichi*; Kawasaki, Takeshi*; Harada, Masayuki*; Ikeda, Yasuhisa*; Kawata, Yoshihisa*; Morita, Yasuji; Kikuchi, Toshiaki*
no journal, ,
We have been developing an advanced reprocessing system for spent FBR fuels based on precipitation method using pyrrolidone derivatives. In the present study, the decomposition of residual precipitants in the solution after precipitation was examined by  -ray irradiation of 9M nitric acid solution containing N-n-butyl-2-pyrrolidone (NBP) which would be used in the first selective U precipitation step. We found that NBP can be easily decomposed by
-ray irradiation of 9M nitric acid solution containing N-n-butyl-2-pyrrolidone (NBP) which would be used in the first selective U precipitation step. We found that NBP can be easily decomposed by  -ray but oxalic acid, acetic acid and other products by the decomposition remain in the solution. Further study is required for the complete decomposition of organic reagents.
-ray but oxalic acid, acetic acid and other products by the decomposition remain in the solution. Further study is required for the complete decomposition of organic reagents.