Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Yanase, Nobuyuki; Naganawa, Hirochika; Nagano, Tetsushi; Noro, Junji*
Analytical Sciences, 27(3), p.325 - 330, 2011/03
Times Cited Count:11 Percentile:33.80(Chemistry, Analytical)JP, 2008-253779 Licensable Patent Information Database
Licensable Patent Information Database A single current "emulsion flow" liquid-liquid extraction apparatus has a head having a number of holes from which micrometer-sized droplets of an aqueous phase spout into an organic phase to mix the two liquid phases. For practical use, however, it is a fatal problem that particulate components in the aqueous phase plug the holes. In the present study, we have succeeded to solve the problem by applying a counter current type emulsion flow apparatus where micrometer-sized droplets of the organic phase are generated.
Naganawa, Hirochika; Suzuki, Hideya*; Yanase, Nobuyuki; Nagano, Tetsushi; Noro, Junji*
Analytical Sciences, 27(3), p.321 - 324, 2011/03
Times Cited Count:10 Percentile:31.17(Chemistry, Analytical)In order to monitor a radioactive nuclide of strontium-90 in seawater around nuclear facilities, a solvent extraction method for collecting Sr(II) in seawater was examined. A reversed-micellar extraction system containing an anionic surfactant AOT and a molecular extractant TODGA in n-hexane was chosen for the extraction of Sr(II) from model solutions of seawater containing 0.5 M NaCl (1 M = 1 mol dm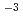 ), 0.05 M MgCl
), 0.05 M MgCl , and/or 0.01 M CaCl
, and/or 0.01 M CaCl . The combination of AOT forming reversed micelles and TODGA coordinating with Sr(II) as an organic ligand (extractant) was found to be efficient for the extraction of Sr(II) from the model solutions. The mechanism of the reversed-micellar extraction system was also discussed in the present study.
. The combination of AOT forming reversed micelles and TODGA coordinating with Sr(II) as an organic ligand (extractant) was found to be efficient for the extraction of Sr(II) from the model solutions. The mechanism of the reversed-micellar extraction system was also discussed in the present study.
Yanase, Nobuyuki; Naganawa, Hirochika; Nagano, Tetsushi; Noro, Junji*
Analytical Sciences, 27(2), p.171 - 174, 2011/02
Times Cited Count:16 Percentile:45.74(Chemistry, Analytical)JP, 2007-136496 Licensable Patent Information Database
Licensable Patent Information Database A simple and low-cost apparatus for continuous and efficient liquid-liquid extraction, which does not need continual mechanical forces (stirring, shaking, etc.) other than solution sending, has newly been developed. This apparatus is composed of a column part where an emulsion state fluid flow (emulsion flow) is generated by spouting micrometer-sized droplets of an aqueous phase into an organic phase and a phase separating part where the emulsion flow is destabilized by means of a sudden decrease in its flow rate. In the present study, the performance of an emulsion flow apparatus in the extraction of Yb(III) and U(VI) from aqueous HNO solutions into isooctane containing D2EHPA was evaluated. The mixing efficiency of the emulsion flow apparatus was found to be comparable with that of a popular liquid-liquid extractor, mixer-settler. Moreover, the emulsion flow apparatus proved to have an overwhelming advantage in terms of phase-separating ability.
solutions into isooctane containing D2EHPA was evaluated. The mixing efficiency of the emulsion flow apparatus was found to be comparable with that of a popular liquid-liquid extractor, mixer-settler. Moreover, the emulsion flow apparatus proved to have an overwhelming advantage in terms of phase-separating ability.
Shimojo, Kojiro; Naganawa, Hirochika; Noro, Junji*; Kubota, Fukiko*; Goto, Masahiro*
Analytical Sciences, 23(12), p.1427 - 1430, 2007/12
Times Cited Count:72 Percentile:88.84(Chemistry, Analytical)The extraction and separation of lanthanides have been investigated using CHON type extractants, which are composed of only C, H, O, and N atoms. N, N-dioctyldiglycol amic acid (DODGAA) shows the high extraction and separation performances for heavier lanthanides compared with typical CHON type extractants. On the other hand, N, N, N', N'-tetrakis(2-pyridylmethyl)ethylenediamine (TPEN) provides the unprecedentedly high selectivity for lighter lanthanides. Furthermore, it was found that the combination of DODGAA and TPEN under suitable conditions enables the mutual separation of light, middle, and heavy lanthanides.
Naganawa, Hirochika; Shimojo, Kojiro; Mitamura, Hisayoshi; Sugo, Yumi; Noro, Junji*; Goto, Masahiro*
Solvent Extraction Research and Development, Japan, 14, p.151 - 159, 2007/00
A compound having diglycol amic acid frame, DODGAA, has been synthesized as a new "green" extractant for lanthanides. The new extractant composed, hydrogen, oxygen, and nitrogen atoms is fully combustible to gases without solid waste and its solubility in water is found to be very low. These properties enable us to avoid the burden of industrial waste produced by the incineration of deteriorated extractant and the risk of aquatic environmental pollution. In the extraction ability for lanthanide ions and the ability for their mutual separation, DODGAA is much superior to carboxykic acid type extractants, such as Versatic10, that can also be completely combustible. The extraction and separation performance of DODGAA is comparable to that of organophosphorus extractants, such as PC-88A and DEHPA, that are widely used in current industry but less "green".
Naganawa, Hirochika; Suzuki, Hideya*; Noro, Junji*; Kimura, Takaumi
Chemical Communications, (23), p.2963 - 2965, 2005/06
A "superweak" anion, TFPB-, gives rise to a field effect on the selectivity for Am over Ln
over Ln in their extraction from aqueous HNO
in their extraction from aqueous HNO solution into benzene containing a "hard donor" extractant that shows no selectivity for these metal ions in traditional solvent extraction.
solution into benzene containing a "hard donor" extractant that shows no selectivity for these metal ions in traditional solvent extraction.
Noro, Junji*; Abe, Yuta; Yamashita, Takuya; Matsushima, Tomohiro*
no journal, ,
no abstracts in English
Abe, Yuta; Nakagiri, Toshio; Yamashita, Takuya; Noro, Junji*; Matsushima, Tomohiro*; Kawakami, Tomohiko*
no journal, ,
no abstracts in English
Naganawa, Hirochika; Yanase, Nobuyuki; Nagano, Tetsushi; Mitamura, Hisayoshi; Noro, Junji*
no journal, ,
no abstracts in English
Naganawa, Hirochika; Shimojo, Kojiro; Noro, Junji*; Goto, Masahiro*
no journal, ,
no abstracts in English