Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Ohashi, Tomonori*; Sakamaki, Tatsuya*; Funakoshi, Kenichi*; Hattori, Takanori; Hisano, Naoki*; Abe, Jun*; Suzuki, Akio*
American Mineralogist, 107(3), p.325 - 335, 2022/03
Times Cited Count:1 Percentile:22.72(Geochemistry & Geophysics)The basaltic glass structure were investigated to 18 GPa using in situ X-ray and neutron diffraction. The O-O coordination number (CN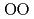 ) starts to rise with maintaining the mean O-O distance (r
) starts to rise with maintaining the mean O-O distance (r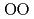 ) above 2-4 GPa, and then CN
) above 2-4 GPa, and then CN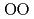 stops increasing and r
stops increasing and r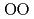 begins to shrink along with the increase in the Al-O coordination number (CN
begins to shrink along with the increase in the Al-O coordination number (CN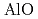 ) above 9 GPa. This is interpreted by the change in the contraction mechanism from tetrahedral network bending to oxygen packing ratio increase via the CN
) above 9 GPa. This is interpreted by the change in the contraction mechanism from tetrahedral network bending to oxygen packing ratio increase via the CN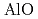 increase. The oxygen packing fraction exceeds the value for dense random packing, suggesting that the oxygen-packing hypothesis cannot account for the pressure-induced structural transformations of silica and silicate glasses. The CN
increase. The oxygen packing fraction exceeds the value for dense random packing, suggesting that the oxygen-packing hypothesis cannot account for the pressure-induced structural transformations of silica and silicate glasses. The CN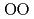 increase at 2-4 GPa reflects the elastic softening of silicate glass, which may causes anomalous elastic moduli of basaltic glass at
increase at 2-4 GPa reflects the elastic softening of silicate glass, which may causes anomalous elastic moduli of basaltic glass at  2 GPa.
2 GPa.