Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Nagoshi, Yasuto*; Fukahori, Takuya*; Okada, Hiroshi*; Takahashi, Akiyuki*; Shimodaira, Masaki; Ueda, Takashi*; Ogawa, Takuya*; Yashirodai, Kenji*; Takahashi, Yukio*; Ohata, Mitsuru*
Transactions of the 27th International Conference on Structural Mechanics in Reactor Technology (SMiRT 27) (Internet), 9 Pages, 2024/03
no abstracts in English
 Cs in spent Cs adsorbent used for the decontamination of radiocesium-containing water by laser ablation inductively coupled plasma mass spectrometry
Cs in spent Cs adsorbent used for the decontamination of radiocesium-containing water by laser ablation inductively coupled plasma mass spectrometryAsai, Shiho*; Ohata, Masaki*; Hanzawa, Yukiko; Horita, Takuma; Yomogida, Takumi; Kitatsuji, Yoshihiro
Analytical Chemistry, 92(4), p.3276 - 3284, 2020/02
Times Cited Count:5 Percentile:28.7(Chemistry, Analytical)The long-term safety assessment of spent Cs adsorbents produced during the decontamination of radiocesium-containing water at the Fukushima Daiichi Nuclear Power Plant requires one to estimate their  Cs content prior to final disposal.
Cs content prior to final disposal.  Cs is usually quantified by inductively coupled plasma mass spectrometry (ICP-MS), which necessitates the elution of Cs from Cs adsorbents. However, this approach suffers from the high radiation dose from
Cs is usually quantified by inductively coupled plasma mass spectrometry (ICP-MS), which necessitates the elution of Cs from Cs adsorbents. However, this approach suffers from the high radiation dose from  Cs. To address this challenge, we herein employed laser ablation ICP-MS for direct quantitation of
Cs. To address this challenge, we herein employed laser ablation ICP-MS for direct quantitation of  Cs in Cs adsorbents and used a model Cs adsorbent prepared by immersion of a commercially available Cs adsorbent into radiocesium-containing liquid waste to verify the developed technique. The use of the
Cs in Cs adsorbents and used a model Cs adsorbent prepared by immersion of a commercially available Cs adsorbent into radiocesium-containing liquid waste to verify the developed technique. The use of the  Cs/
Cs/ Cs ratio and
Cs ratio and  Cs radioactivity obtained by gamma spectrometry achieved simple and precise quantitation of
Cs radioactivity obtained by gamma spectrometry achieved simple and precise quantitation of  Cs and the resulting
Cs and the resulting  Cs activity of 0.36 Bq agreed well with that in the original radiocesium-containing liquid waste.
Cs activity of 0.36 Bq agreed well with that in the original radiocesium-containing liquid waste.
 Pd in Pd purified by selective precipitation from spent nuclear fuel by laser ablation ICP-MS
Pd in Pd purified by selective precipitation from spent nuclear fuel by laser ablation ICP-MSAsai, Shiho; Ohata, Masaki*; Yomogida, Takumi; Saeki, Morihisa*; Oba, Hironori*; Hanzawa, Yukiko; Horita, Takuma; Kitatsuji, Yoshihiro
Analytical and Bioanalytical Chemistry, 411(5), p.973 - 983, 2019/02
Times Cited Count:11 Percentile:60.2(Biochemical Research Methods)Determination of radiopalladium  Pd is required for ensuring the radiation safety of Pd extracted from spent nuclear fuel for recycling or disposal. We employed laser ablation inductively coupled plasma mass spectrometry (LA-ICP-MS) to simplify an analytical procedure of
Pd is required for ensuring the radiation safety of Pd extracted from spent nuclear fuel for recycling or disposal. We employed laser ablation inductively coupled plasma mass spectrometry (LA-ICP-MS) to simplify an analytical procedure of  Pd. Pd was separated through selective Pd precipitation reaction from spent nuclear fuel. Laser ablation allows direct measurement of the Pd precipitates, skipping the dissolution and dilution procedure. In this study,
Pd. Pd was separated through selective Pd precipitation reaction from spent nuclear fuel. Laser ablation allows direct measurement of the Pd precipitates, skipping the dissolution and dilution procedure. In this study, 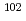 Pd in natural Pd standard solution was used as an internal standard, taking advantage of its absence in spent nuclear fuel. The Pd precipitate was uniformly embedded on the surface of the centrifugal filter, forming a microscopically thin flat surface of Pd. The resulting homogeneous Pd layer is suitable for obtaining a stable signal ratio of
Pd in natural Pd standard solution was used as an internal standard, taking advantage of its absence in spent nuclear fuel. The Pd precipitate was uniformly embedded on the surface of the centrifugal filter, forming a microscopically thin flat surface of Pd. The resulting homogeneous Pd layer is suitable for obtaining a stable signal ratio of  Pd/
Pd/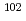 Pd. The amount of
Pd. The amount of  Pd obtained by LA-ICP-MS corresponds to the values obtained by conventional solution nebulization measurement.
Pd obtained by LA-ICP-MS corresponds to the values obtained by conventional solution nebulization measurement.
Tanabe, Tetsuo*; Sugiyama, Kazuyoshi*; Shibahara, Takahiro*; Hirohata, Yuko*; Yoshida, Masafumi; Masaki, Kei; Sato, Masayasu
Journal of Nuclear Materials, 390-391, p.705 - 708, 2009/06
Times Cited Count:8 Percentile:49.52(Materials Science, Multidisciplinary)no abstracts in English
Masaki, Kei; Tanabe, Tetsuo*; Hirohata, Yuko*; Oya, Yasuhisa*; Shibahara, Takahiro*; Hayashi, Takao; Sugiyama, Kazuyoshi*; Arai, Takashi; Okuno, Kenji*; Miya, Naoyuki
Nuclear Fusion, 47(11), p.1577 - 1582, 2007/11
Times Cited Count:14 Percentile:45.18(Physics, Fluids & Plasmas)In JT-60U, erosion/deposition analyses for the plasma facing wall have shown that deposition was dominant at the inner-middle first wall and the inner divertor, whereas erosion dominant at the upper first wall and the outer divertor. Assuming toroidal symmetry in the erosion and deposition patterns, the net carbon erosion and deposition in the divertor area were estimated to be 0.34 kg and 0.55 kg, respectively. In a whole, the increment of carbon in the divertor region was 0.21 kg, which should be originated from the first wall. The hydrogen concentration in the thick deposition layer of the inner divertor was 0.02 in (H+D)/C. In the plasma-shadowed area underneath the divertor region at around 420 K, re-deposited layers of 2  m-thick were found with high hydrogen concentration of 0.8 in (H+D)/C. The carbon deposition rate in the plasma-shadowed area, however, was 8
m-thick were found with high hydrogen concentration of 0.8 in (H+D)/C. The carbon deposition rate in the plasma-shadowed area, however, was 8 10
10 atoms/s, which was one order smaller than that (6
atoms/s, which was one order smaller than that (6 10
10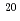 atoms/s) on the wall surface.
atoms/s) on the wall surface.
Oya, Yasuhisa*; Hirohata, Yuko*; Nakahata, Toshihiko*; Suda, Taichi*; Yoshida, Masashi*; Arai, Takashi; Masaki, Kei; Okuno, Kenji*; Tanabe, Tetsuo*
Fusion Science and Technology, 52(3), p.554 - 558, 2007/10
Times Cited Count:0 Percentile:0.01(Nuclear Science & Technology)To investigate retention characteristics of hydrogen isotopes in the first wall tiles of JT-60U, surface morphology, erosion/deposition profiles and hydrogen isotope retentions were examined by SEM, XPS, TDS and SIMS. It was found that poloidal deuterium retention profile was rather uniform, while the thermal desorption behavior of deuterium was quite different depending on the locations of the tiles. Deuterium retained in the upper first wall, where was covered by thick boron layers with high concentration of B, was desorbed at lower temperature than that in the lower area covered by carbon layers with much less B content. D/H ratio in the first wall tiles was appreciably higher than that observed in the divertor tiles, suggesting the injection of high energy deuteron originating from NBI into the first wall. In addition, the lower temperature of the first wall compared to that of the divertor tiles would prohibit desorption of the implanted deuterium and/or its replacement by subsequent D or H impingement.
Hirohata, Yuko*; Tanabe, Tetsuo*; Oya, Yasuhisa*; Okuno, Kenji*; Masaki, Kei; Miya, Naoyuki; JT-60U Team
Journal of Nuclear Materials, 363-365, p.854 - 861, 2007/06
Times Cited Count:11 Percentile:61.1(Materials Science, Multidisciplinary)no abstracts in English
Masaki, Kei; Tanabe, Tetsuo*; Hirohata, Yuko*; Oya, Yasuhisa*; Shibahara, Takahiro*; Hayashi, Takao; Sugiyama, Kazuyoshi*; Arai, Takashi; Okuno, Kenji*; Miya, Naoyuki
Proceedings of 21st IAEA Fusion Energy Conference (FEC 2006) (CD-ROM), 8 Pages, 2007/03
Evaluation of fuel inventory and its retention process are critical issues for a next-step fusion device, especially with carbon-based wall. In order to resolve the issues, the hydrogen retention and carbon deposition analyses for the plasma facing surfaces and plasma-shadowed area of JT-60U have been performed. In JT-60U, erosion/deposition analyses for the plasma facing wall have shown that deposition was dominant at the inner-middle first wall and the inner divertor, whereas erosion dominant at the upper first wall and the outer divertor. Assuming toroidal symmetry in the erosion and deposition patterns, the net carbon erosion and deposition in the divertor area were estimated to be 0.34 kg and 0.55 kg, respectively. In a whole, the increment of carbon in the divertor region was 0.21 kg, which should be originated from the first wall. The hydrogen concentration in the thick deposition layer of the inner divertor was  0.02 in (H+D)/C. In the plasma-shadowed area underneath the divertor region at around 420 K, re-deposited layers of
0.02 in (H+D)/C. In the plasma-shadowed area underneath the divertor region at around 420 K, re-deposited layers of  2
2 m-thick were found with high hydrogen concentration of
m-thick were found with high hydrogen concentration of  0.8 in (H+D)/C. The carbon deposition rate in the plasma-shadowed area, however, was 8
0.8 in (H+D)/C. The carbon deposition rate in the plasma-shadowed area, however, was 8 10
10 atoms/s, which was one order smaller than that (6
atoms/s, which was one order smaller than that (6 10
10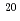 atoms/s) on the wall surface.
atoms/s) on the wall surface.
Shibahara, Takahiro*; Tanabe, Tetsuo*; Hirohata, Yuko*; Oya, Yasuhisa*; Oyaizu, Makoto*; Yoshikawa, Akira*; Onishi, Yoshihiro*; Arai, Takashi; Masaki, Kei; Okuno, Kenji*; et al.
Journal of Nuclear Materials, 357(1-3), p.115 - 125, 2006/10
Times Cited Count:20 Percentile:78.83(Materials Science, Multidisciplinary)no abstracts in English
Shibahara, Takahiro*; Tanabe, Tetsuo*; Hirohata, Yuko*; Oya, Yasuhisa*; Oyaizu, Makoto*; Yoshikawa, Akira*; Onishi, Yoshihiro*; Arai, Takashi; Masaki, Kei; Okuno, Kenji*; et al.
Nuclear Fusion, 46(10), p.841 - 847, 2006/10
Times Cited Count:18 Percentile:52.46(Physics, Fluids & Plasmas)no abstracts in English
Oya, Yasuhisa*; Hirohata, Yuko*; Tanabe, Tetsuo*; Shibahara, Takahiro*; Kimura, Hiromi*; Oyaizu, Makoto*; Arai, Takashi; Masaki, Kei; Goto, Yoshitaka*; Okuno, Kenji*; et al.
Fusion Engineering and Design, 75-79, p.945 - 949, 2005/11
Times Cited Count:9 Percentile:53.19(Nuclear Science & Technology)no abstracts in English
Hirohata, Yuko*; Shibahara, Takahiro*; Tanabe, Tetsuo*; Oya, Yasuhisa*; Arai, Takashi; Goto, Yoshitaka*; Masaki, Kei; Yagyu, Junichi; Oyaizu, Makoto*; Okuno, Kenji*; et al.
Fusion Science and Technology, 48(1), p.557 - 560, 2005/07
Times Cited Count:3 Percentile:24.22(Nuclear Science & Technology)no abstracts in English
Masaki, Kei; Sugiyama, Kazuyoshi*; Hayashi, Takao; Ochiai, Kentaro; Goto, Yoshitaka*; Shibahara, Takahiro*; Hirohata, Yuko*; Oya, Yasuhisa*; Miya, Naoyuki; Tanabe, Tetsuo*
Journal of Nuclear Materials, 337-339, p.553 - 559, 2005/03
Times Cited Count:26 Percentile:84.04(Materials Science, Multidisciplinary)no abstracts in English
Hirohata, Yuko*; Shibahara, Takahiro*; Tanabe, Tetsuo*; Arai, Takashi; Goto, Yoshitaka*; Oya, Yasuhisa*; Yoshida, Hajime*; Morimoto, Yasutomi*; Yagyu, Junichi; Masaki, Kei; et al.
Journal of Nuclear Materials, 337-339, p.609 - 613, 2005/03
Times Cited Count:13 Percentile:65.42(Materials Science, Multidisciplinary)no abstracts in English
Oya, Yasuhisa*; Hirohata, Yuko*; Morimoto, Yasutomi*; Yoshida, Hajime*; Kodama, Hiroshi*; Kizu, Kaname; Yagyu, Junichi; Goto, Yoshitaka*; Masaki, Kei; Okuno, Kenji*; et al.
Journal of Nuclear Materials, 313-316, p.209 - 213, 2003/03
Times Cited Count:25 Percentile:82.89(Materials Science, Multidisciplinary)no abstracts in English
Shibahara, Takahiro*; Hirohata, Yuko*; Oya, Yasuhisa*; Oyaizu, Makoto*; Onishi, Yoshihiro*; Yoshikawa, Akira*; Okuno, Kenji*; Sugiyama, Kazuyoshi*; Tanabe, Tetsuo*; Arai, Takashi; et al.
no journal, ,
no abstracts in English
Hirohata, Yuko*; Tanabe, Tetsuo*; Sugiyama, Kazuyoshi*; Shibahara, Takahiro*; Oya, Yasuhisa*; Oyaizu, Makoto*; Yoshikawa, Akira*; Okuno, Kenji*; Masaki, Kei; Arai, Takashi; et al.
no journal, ,
no abstracts in English
Hirohata, Yuko*; Tanabe, Tetsuo*; Oya, Yasuhisa*; Shibahara, Takahiro*; Sugiyama, Kazuyoshi*; Oyaizu, Makoto*; Yoshikawa, Akira*; Yoshida, Masashi*; Arai, Takashi; Masaki, Kei; et al.
no journal, ,
no abstracts in English
Hirohata, Yuko*; Tanabe, Tetsuo*; Oya, Yasuhisa*; Shibahara, Takahiro*; Sugiyama, Kazuyoshi*; Oyaizu, Makoto*; Yoshikawa, Akira*; Yoshida, Masashi*; Arai, Takashi; Masaki, Kei; et al.
no journal, ,
no abstracts in English
Hirohata, Yuko*; Tanabe, Tetsuo*; Oya, Yasuhisa*; Arai, Takashi; Masaki, Kei; Okuno, Kenji*; Sugiyama, Kazuyoshi*; Oyaizu, Makoto*; Yoshikawa, Akira*; Yoshida, Masashi*
no journal, ,
no abstracts in English