Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
 Be
Be O
O for high-functional tritium breeders
for high-functional tritium breedersHoshino, Tsuyoshi; Oikawa, Fumiaki; Natori, Yuri*; Kato, Kenichi*; Sakka, Tomoko*; Nakamura, Mutsumi*; Tatenuma, Katsuyoshi*
Fusion Engineering and Design, 88(9-10), p.2268 - 2271, 2013/10
Times Cited Count:1 Percentile:10.69(Nuclear Science & Technology)Lithium titanate with additional Li (Li TiO
TiO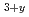 ) and lithium orthosilicate (Li
) and lithium orthosilicate (Li SiO
SiO ) is one of the most promising candidates for use in a tritium breeder because of its good chemical and mechanical stabilities. Currently, mixtures of tritium breeder pebble and neutron multiplier (Be or Be
) is one of the most promising candidates for use in a tritium breeder because of its good chemical and mechanical stabilities. Currently, mixtures of tritium breeder pebble and neutron multiplier (Be or Be Ti) pebble are being considered for use in increasing the tritium breeding ratio in a breeding blanket. However, lithium and beryllium are gradually reacted under practical operating conditions, and therefore a high-functional tritium breeder such as lithium beryllium oxide (Li
Ti) pebble are being considered for use in increasing the tritium breeding ratio in a breeding blanket. However, lithium and beryllium are gradually reacted under practical operating conditions, and therefore a high-functional tritium breeder such as lithium beryllium oxide (Li Be
Be O
O ) needs to be developed to compensate for this reactive characteristic under high temperature use. In this study, methods of synthesizing Li
) needs to be developed to compensate for this reactive characteristic under high temperature use. In this study, methods of synthesizing Li Be
Be O
O have been extensively investigated by means of solid-phase reaction. The solid-phase reaction of LiOH(H
have been extensively investigated by means of solid-phase reaction. The solid-phase reaction of LiOH(H O) and BeO is a suitable synthesis method for lithium beryllium oxide (Li
O) and BeO is a suitable synthesis method for lithium beryllium oxide (Li Be
Be O
O ). It is expected that single-phase Li
). It is expected that single-phase Li Be
Be O
O will be stable under the mixture conditions of a tritium breeder and neutron multiplier in the blanket region at high temperatures.
will be stable under the mixture conditions of a tritium breeder and neutron multiplier in the blanket region at high temperatures.
Hoshino, Tsuyoshi; Oikawa, Fumiaki
Fusion Engineering and Design, 86(9-11), p.2172 - 2175, 2011/10
Times Cited Count:37 Percentile:93.02(Nuclear Science & Technology)Lithium titanate (Li TiO
TiO ) is one of the most promising candidates among tritium breeding materials because of its good tritium release characteristics. However, the mass of Li
) is one of the most promising candidates among tritium breeding materials because of its good tritium release characteristics. However, the mass of Li TiO
TiO decreased with time in a hydrogen atmosphere by Li evaporation and with Li burn up. In order to prevent the mass decrease at high temperatures, Li
decreased with time in a hydrogen atmosphere by Li evaporation and with Li burn up. In order to prevent the mass decrease at high temperatures, Li TiO
TiO with added Li have been developed as one of advanced tritium breeders. We have been promoting the development of fabrication technique of Li
with added Li have been developed as one of advanced tritium breeders. We have been promoting the development of fabrication technique of Li TiO
TiO pebbles by the sol-gel method. The fabrication techniques of advanced tritium breeder pebbles have not been established for large quantities. Therefore, trial fabrication tests of advanced breeder pebbles were carried out using previous sol-gel method. The diameter of the pebbles is 1.18 mm, and the sphericity is 1.04. It is expected that an advanced tritium breeder with added Li will be stable under operating conditions, namely in a neutron environment at a high temperatures. Thus, these results show that the pebble fabrication using the sol-gel method is a promising production technique for mass production of the advanced tritium breeder pebbles.
pebbles by the sol-gel method. The fabrication techniques of advanced tritium breeder pebbles have not been established for large quantities. Therefore, trial fabrication tests of advanced breeder pebbles were carried out using previous sol-gel method. The diameter of the pebbles is 1.18 mm, and the sphericity is 1.04. It is expected that an advanced tritium breeder with added Li will be stable under operating conditions, namely in a neutron environment at a high temperatures. Thus, these results show that the pebble fabrication using the sol-gel method is a promising production technique for mass production of the advanced tritium breeder pebbles.
Hoshino, Tsuyoshi; Kato, Kenichi*; Natori, Yuri*; Oikawa, Fumiaki; Nakano, Natsuko*; Nakamura, Mutsumi*; Sasaki, Kazuya*; Suzuki, Akihiro*; Terai, Takayuki*; Tatenuma, Katsuyoshi*
Journal of Nuclear Materials, 417(1-3), p.684 - 687, 2011/10
Times Cited Count:52 Percentile:96.13(Materials Science, Multidisciplinary)Lithium titanate (Li TiO
TiO ) is one of the most promising candidates among tritium breeding materials because of its good tritium release. Addition of H
) is one of the most promising candidates among tritium breeding materials because of its good tritium release. Addition of H to inert sweep gas has been proposed for enhancing the tritium release from tritium breeding materials. However, the mass of Li
to inert sweep gas has been proposed for enhancing the tritium release from tritium breeding materials. However, the mass of Li TiO
TiO was decreased with time in the hydrogen atmosphere. It is assumed that the mass decrease indicates the loss of the oxygen contained in the sample caused by the change from Ti
was decreased with time in the hydrogen atmosphere. It is assumed that the mass decrease indicates the loss of the oxygen contained in the sample caused by the change from Ti  to Ti
to Ti  , and that the partial pressures of Li-containing species were increased in the hydrogen atmosphere. In order to decrease the mass-change at high temperature, advanced tritium breeding material with added Li should be developed to improve the physical and chemical stability in hydrogen atmosphere. In the case of the Li
, and that the partial pressures of Li-containing species were increased in the hydrogen atmosphere. In order to decrease the mass-change at high temperature, advanced tritium breeding material with added Li should be developed to improve the physical and chemical stability in hydrogen atmosphere. In the case of the Li TiO
TiO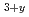 samples used by the present study, LiOHH
samples used by the present study, LiOHH O and H
O and H TiO
TiO were proportionally mixed with the molar ratio Li/Ti of either 2.0 and 2.2. These samples are designated as L20 (Li/Ti = 2.0) and L22 (Li/Ti = 2.2), respectively. The results of XRD measurement showed that the phases in advanced tritium breeding material were as follows. L22 existed as non-stoichiometric compound Li
were proportionally mixed with the molar ratio Li/Ti of either 2.0 and 2.2. These samples are designated as L20 (Li/Ti = 2.0) and L22 (Li/Ti = 2.2), respectively. The results of XRD measurement showed that the phases in advanced tritium breeding material were as follows. L22 existed as non-stoichiometric compound Li TiO
TiO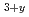 .
.
 Be
Be O
O for high-functional tritium breeder
for high-functional tritium breederHoshino, Tsuyoshi; Natori, Yuri*; Sakka, Tomoko*; Kato, Kenichi*; Oikawa, Fumiaki; Nakamura, Mutsumi*; Tatenuma, Katsuyoshi*
no journal, ,
no abstracts in English
Oikawa, Fumiaki; Kim, Jae-Hwan; Yonehara, Kazuo; Wakai, Daisuke; Nakamichi, Masaru
no journal, ,
no abstracts in English