Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
 O
O determined by aerodynamic levitation
determined by aerodynamic levitationSun, Y.*; Takatani, Tomoya*; Muta, Hiroaki*; Fujieda, Shun*; Kondo, Toshiki; Kikuchi, Shin; Kargl, F.*; Oishi, Yuji*
International Journal of Thermophysics, 45(1), p.11_1 - 11_19, 2024/01
Times Cited Count:1 Percentile:35.22(Thermodynamics)no abstracts in English
Kinoshita, Ryoma; Sasaki, Yuji; Kaneko, Masashi; Matsumiya, Masahiko*; Shinoku, Kota*; Shiroishi, Hidenobu*
Hydrometallurgy, 222, p.106159_1 - 106159_12, 2023/10
Times Cited Count:2 Percentile:23.52(Metallurgy & Metallurgical Engineering)Solvent extraction is conducted using a total of 20 metals revealing high stability constants with Cl and hexahexyl-nitrilotriacetamide (NTAamide(C6)) extractant. The metals used here may behave as anions at high Cl concentrations, and NTAamide(C6), which contains a tertiary N atom, is protonated under acidic conditions. Most of the metal ions in this study display higher distribution ratios (D(M)) from HCl than those from HNO , and exhibit 1:1 stoichiometries with NTAamide. Following the experimental results, the association constants and distribution coefficients of the group 12 elements are calculated via ion-pair extraction modeling using density functional theory calculations, and the simulations of D yield calculated values with the same trend as that of the measured values.
, and exhibit 1:1 stoichiometries with NTAamide. Following the experimental results, the association constants and distribution coefficients of the group 12 elements are calculated via ion-pair extraction modeling using density functional theory calculations, and the simulations of D yield calculated values with the same trend as that of the measured values.
Sasaki, Yuji; Kaneko, Masashi; Ban, Yasutoshi; Kinoshita, Ryoma; Matsumiya, Masahiko*; Shinoku, Kota*; Shiroishi, Hidenobu*
Analytical Sciences, 39(9), p.1575 - 1583, 2023/09
Times Cited Count:3 Percentile:35.90(Chemistry, Analytical)Extraction of Rh from HCl can be performed by NTAamide(C6) (hexahexyl-nitrilotriacetamide) and other related compounds into n-dodecane. We use ion-pair extraction of anionic species of Rh-chloride and protonated extractant. Rh behave as anion in hydrochloric acid and the tertiary nitrogen atom in extractant may be protonated to produce the quaternary amine in acidic condition. From the present work, the maximum distribution ratio of Rh(III) is 16. The D(Rh) values are changeable during preparation of the aqueous solutions because different Rh-Cl-H O complexes are formed in HCl media and show the slow exchange rate between Cl and H
O complexes are formed in HCl media and show the slow exchange rate between Cl and H O. Using the UV spectrum, Rh-chloride solution having the peak of spectrum at 504 nm can be extracted effectively, where RhCl
O. Using the UV spectrum, Rh-chloride solution having the peak of spectrum at 504 nm can be extracted effectively, where RhCl (H
(H O)
O) and RhCl
and RhCl (H
(H O)
O)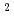
 exist mainly from DFT calculation. Stoichiometry of one-one complex of Rh and NTAamide is obtained from slope analysis, and 85 mM of concentrated Rh ion can be extracted.
exist mainly from DFT calculation. Stoichiometry of one-one complex of Rh and NTAamide is obtained from slope analysis, and 85 mM of concentrated Rh ion can be extracted.
 O
O )
)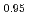 -(SiO
-(SiO )
)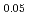 measured by aerodynamic levitation
measured by aerodynamic levitationKondo, Toshiki; Toda, Taro*; Takeuchi, Junichi*; Kikuchi, Shin; Kargl, F.*; Muta, Hiroaki*; Oishi, Yuji*
High Temperatures-High Pressures, 52(3-4), p.307 - 321, 2023/06
Times Cited Count:0 Percentile:0.00(Thermodynamics)In order to establish an evaluation method/numerical simulation for nuclear reactor safety under severe accidental conditions, it is necessary to obtain the physical properties, especially fluidity of the relevant molten materials at very high temperatures. In this study, thermophysical properties such as density and viscosity were obtained for (Fe O
O )
)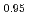 -(SiO
-(SiO )
)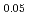 , which is a representative composition in the early stage of severe accident. (Fe
, which is a representative composition in the early stage of severe accident. (Fe O
O )
)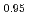 -(SiO
-(SiO )
)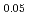 is produced by the contact between the molten oxide of steel, which is the main component of the reactor, and SiO
is produced by the contact between the molten oxide of steel, which is the main component of the reactor, and SiO , which is the main component of concrete. As a result, the physical properties of the (Fe
, which is the main component of concrete. As a result, the physical properties of the (Fe O
O )
)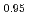 -(SiO
-(SiO )
)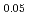 mixture were almost the same as those of Fe
mixture were almost the same as those of Fe O
O obtained in previous studies, and it could be concluded that a small amount of SiO
obtained in previous studies, and it could be concluded that a small amount of SiO (about 5 mol.%) did not significantly affect the fluidity of Fe
(about 5 mol.%) did not significantly affect the fluidity of Fe O
O .
.
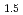 , (FeO
, (FeO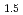 )
)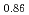 -(ZrO
-(ZrO )
)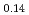 and (FeO
and (FeO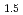 )
)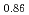 -(UO
-(UO )
)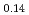
Kondo, Toshiki; Toda, Taro*; Takeuchi, Junichi*; Kargl, F.*; Kikuchi, Shin; Muta, Hiroaki*; Oishi, Yuji*
Journal of Nuclear Science and Technology, 59(9), p.1139 - 1148, 2022/09
Times Cited Count:1 Percentile:14.04(Nuclear Science & Technology)no abstracts in English
Sasaki, Yuji; Morita, Keisuke; Matsumiya, Masahiko*; Ono, Ryoma*; Shiroishi, Hidenobu*
JOM, 73(4), p.1037 - 1043, 2021/04
Times Cited Count:4 Percentile:28.73(Materials Science, Multidisciplinary)The separation of Dy from Nd is studied from the viewpoint of recycling Dy from Nd magnets. Both metals are lanthanide elements, which means their mutual separation is difficult because of their similar chemical behaviors. All lanthanide elements can be extracted easily by using tetradodecyl-diglycolamide (TDdDGA) extractants, and it has a relatively high separation factor (SF) between Dy and Nd (SF over 10). In the present study, by performing eight extraction steps with the organic phase (0.1M TDdDGA in dodecane), ten steps with an aqueous phase (0.7 M HNO with metals), and six steps with another aqueous phase (0.7 M HNO
with metals), and six steps with another aqueous phase (0.7 M HNO without metals), approximately 99% Dy was recovered into the organic phase with 1% co-extraction of Nd.
without metals), approximately 99% Dy was recovered into the organic phase with 1% co-extraction of Nd.
Tsuchikawa, Yusuke; Abe, Yuta; Oishi, Yuji*; Kai, Tetsuya; Toh, Yosuke; Segawa, Mariko; Maeda, Makoto; Kimura, Atsushi; Nakamura, Shoji; Harada, Masahide; et al.
JPS Conference Proceedings (Internet), 33, p.011074_1 - 011074_6, 2021/03
In the decommissioning of the Fukushima-Daiichi (1F) Nuclear Power Plant, it is essential to understand characteristics of the melted core materials. The estimation of boride in the real debris is of great importance to develop safe debris removal plans. Hence, it is required to investigate the amount of boron in the melted core materials with nondestructive methods. Prompt gamma-ray activation analysis (PGAA) is one of the useful techniques to determine the amount of borides by means of the 478 keV prompt gamma-ray from neutron absorption reaction of boron. Moreover, it is well known that the width of the 478 keV gamma-ray peak is typically broadened due to the Doppler effect. The degree of the broadening is affected by coexisting materials, and can be recognized by the width of the prompt gamma-ray peak. As a feasibility study, the prompt gamma-ray from boride samples were measured using the ANNRI, NOBORU, and RADEN beamlines at the Materials and Life Science Experimental Facility (MLF) of Japan Proton Accelerator Complex (J-PARC).
Abe, Yuta; Tsuchikawa, Yusuke; Kai, Tetsuya; Matsumoto, Yoshihiro*; Parker, J. D.*; Shinohara, Takenao; Oishi, Yuji*; Kamiyama, Takashi*; Nagae, Yuji; Sato, Ikken
JPS Conference Proceedings (Internet), 33, p.011075_1 - 011075_6, 2021/03
Tsuchikawa, Yusuke; Kai, Tetsuya; Abe, Yuta; Oishi, Yuji*; Sun, Y.*; Oikawa, Kenichi; Nakatani, Takeshi; Sato, Ikken
Nuclear Instruments and Methods in Physics Research A, 991, p.164964_1 - 164964_5, 2021/03
Times Cited Count:1 Percentile:13.22(Instruments & Instrumentation)Peak shape analysis was performed for the energy spectra of Doppler-broadened prompt  -rays generated by neutron capture reactions with various boride or boron samples. Significant differences were observed between nonmetallic and metallic borides. Minor differences between the peak shapes of prompt
-rays generated by neutron capture reactions with various boride or boron samples. Significant differences were observed between nonmetallic and metallic borides. Minor differences between the peak shapes of prompt  -rays from zirconium- and ferro-borons were evaluated by a peak fitting method. The identification of zirconium- and ferro-borons and other types of borides was estimated.
-rays from zirconium- and ferro-borons were evaluated by a peak fitting method. The identification of zirconium- and ferro-borons and other types of borides was estimated.
Sun, Y.*; Abe, Yuta; Muta, Hiroaki*; Oishi, Yuji*
Journal of Nuclear Science and Technology, 57(8), p.917 - 925, 2020/08
Times Cited Count:5 Percentile:42.20(Nuclear Science & Technology)Abe, Yuta; Tsuchikawa, Yusuke; Kai, Tetsuya; Matsumoto, Yoshihiro*; Parker, J. D.*; Shinohara, Takenao; Oishi, Yuji*; Kamiyama, Takashi*; Nagae, Yuji; Sato, Ikken
Proceedings of 2020 International Conference on Nuclear Engineering (ICONE 2020) (Internet), 6 Pages, 2020/08
 Si
Si O
O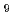
Suzuki, Eriko; Nakajima, Kunihisa; Osaka, Masahiko; Oishi, Yuji*; Muta, Hiroaki*; Kurosaki, Ken*
Journal of Nuclear Science and Technology, 57(7), p.852 - 857, 2020/07
Times Cited Count:5 Percentile:42.20(Nuclear Science & Technology)The low temperature heat capacity of Cs Si
Si O
O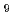 , which is one of the cesium chemisorbed compounds onto stainless steel during severe accident of the light water nuclear reactor, was experimentally determined for the first time in the temperature range of 1.9 - 302 K. The experimentally determined heat capacity,
, which is one of the cesium chemisorbed compounds onto stainless steel during severe accident of the light water nuclear reactor, was experimentally determined for the first time in the temperature range of 1.9 - 302 K. The experimentally determined heat capacity, 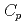
 (298.15K), and the standard entropy,
(298.15K), and the standard entropy, 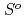 (298.15K), were 249.4
(298.15K), were 249.4  1.1 J K
1.1 J K mol
mol and 322.1
and 322.1  1.3 J K
1.3 J K mol
mol , respectively. The standard Gibbs energy of formation of Cs
, respectively. The standard Gibbs energy of formation of Cs Si
Si O
O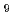 at high temperatures,
at high temperatures, 
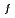
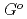 (
(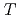 ), were reevaluated by using the presently obtained
), were reevaluated by using the presently obtained 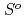 (298.15K) and the previously reported experimental results of the standard enthalpy of formation,
(298.15K) and the previously reported experimental results of the standard enthalpy of formation, 
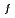
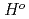 (298.15K), and the standard enthalpy increments at high temperatures,
(298.15K), and the standard enthalpy increments at high temperatures, 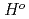 (
(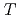 )-
)-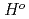 (298.15K).
(298.15K).
Sasaki, Yuji; Ban, Yasutoshi; Morita, Keisuke; Matsumiya, Masahiko*; Ono, Ryoma*; Shiroishi, Hidenobu*
Solvent Extraction Research and Development, Japan, 27(1), p.63 - 67, 2020/00
Times Cited Count:6 Percentile:23.87(Chemistry, Multidisciplinary)Mutual separation technique of Dy and Nd in Nd magnet is studied. Dy is more valuable than Nd, then Dy might be isolated and reused. Lanthanide elements can be extracted thoroughly by diglycolamide (DGA) extractants, we use this reagent for the recovery and isolation of Dy. Tetradodecyl-DGA (TDdDGA) has relatively high separation factors(SF) between Dy and Nd (SF=17-18) in HNO extraction system, counter-current extraction using TDdDGA was applied for their mutual separation. From the present study, using the condition, four extraction stages, organic phase: 0.1M TDdDGA in n-dodecane, aqueous phase: 0.3M HNO
extraction system, counter-current extraction using TDdDGA was applied for their mutual separation. From the present study, using the condition, four extraction stages, organic phase: 0.1M TDdDGA in n-dodecane, aqueous phase: 0.3M HNO , 92% Dy can be recovered with 0.7% co-extraction of Nd.
, 92% Dy can be recovered with 0.7% co-extraction of Nd.
Mohamad, A.*; Nakajima, Kunihisa; Suzuki, Eriko; Miwa, Shuhei; Osaka, Masahiko; Oishi, Yuji*; Muta, Hiroaki*; Kurosaki, Ken*
Proceedings of International Topical Workshop on Fukushima Decommissioning Research (FDR 2019) (Internet), 4 Pages, 2019/05
In the accident of Fukushima Daiichi Nuclear Power Station, formation of a volatile SrCl could have occurred by the sea-water injection into the core. This can cause the release of non-volatile group Sr from the fuel to induce chemical reactions with reactor structural materials, such as stainless steel and Zircaloy (Zry) cladding. Such reactions could cause the changes in distribution of Sr in the reactor. Chemical reactions between Sr species and Zry were therefore investigated experimentally. As the result, it can be said that Sr vapor species were chemically trapped right after the release from fuel. This trapping effect of Sr by Zry-cladding implies a possibility of preferable Sr retention in the oxide phase of debris.
could have occurred by the sea-water injection into the core. This can cause the release of non-volatile group Sr from the fuel to induce chemical reactions with reactor structural materials, such as stainless steel and Zircaloy (Zry) cladding. Such reactions could cause the changes in distribution of Sr in the reactor. Chemical reactions between Sr species and Zry were therefore investigated experimentally. As the result, it can be said that Sr vapor species were chemically trapped right after the release from fuel. This trapping effect of Sr by Zry-cladding implies a possibility of preferable Sr retention in the oxide phase of debris.
 -based simulated fuel containing CsI
-based simulated fuel containing CsITakamatsu, Yuki*; Ishii, Hiroto*; Oishi, Yuji*; Muta, Hiroaki*; Yamanaka, Shinsuke*; Suzuki, Eriko; Nakajima, Kunihisa; Miwa, Shuhei; Osaka, Masahiko; Kurosaki, Ken*
Nihon Genshiryoku Gakkai Wabun Rombunshi, 17(3/4), p.106 - 110, 2018/12
In order to establish the synthesis method of simulated fuel contacting Cesium (Cs) which is required for the evaluation of physical/chemical characteristics in fuel and release behavior of Cs, sintering tests of the cerium dioxide (CeO ) based simulated fuels containing Cesium iodide (CsI) are performed by using spark plasma sintering (SPS) method. The sintered CeO
) based simulated fuels containing Cesium iodide (CsI) are performed by using spark plasma sintering (SPS) method. The sintered CeO pellets with homogeneous distribution of several micro meter of CsI spherical precipitates were successfully obtained by optimizing SPS conditions.
pellets with homogeneous distribution of several micro meter of CsI spherical precipitates were successfully obtained by optimizing SPS conditions.
Muta, Hiroaki*; Nishikane, Ryoji*; Ando, Yusuke*; Matsunaga, Junji*; Sakamoto, Kan*; Harjo, S.; Kawasaki, Takuro; Oishi, Yuji*; Kurosaki, Ken*; Yamanaka, Shinsuke*
Journal of Nuclear Materials, 500, p.145 - 152, 2018/03
Times Cited Count:17 Percentile:79.90(Materials Science, Multidisciplinary)Sasaki, Yuji; Yoshimitsu, Ryo*; Nishihama, Shohei*; Shimbori, Yuma*; Shiroishi, Hidenobu*
Separation Science and Technology, 52(7), p.1186 - 1192, 2017/03
Times Cited Count:2 Percentile:8.14(Chemistry, Multidisciplinary)The new extractant, biuret(C8), is synthesized and tested for the solvent extraction of hard acid metals like actinides and soft acid metals. This compound has the similar central frame to malonamide but with 2-NH introduced into the central frame, then both amidic oxygen and nitrogen atoms may bond with metals. From the present work, not only hard acid metals, but also soft acid metals can be extracted by biuret(C8) from nitric or perchloric acids to n-dodecane. The extractability for biuret(C8) is compared with other representative extractants, malonamide, TODGA and MIDOA. It is clear that the distribution ratio(D) of U and Pu by biuret(C8) is similar to those for malonamide, but with lower values than those for TODGA and MIDOA, and D for soft acid metals and oxonium anions show higher values than those for TODGA and malonamide and lower than those for MIDOA.
Nishiuchi, Mamiko; Choi, I. W.*; Daido, Hiroyuki; Nakamura, Tatsufumi*; Pirozhkov, A. S.; Yogo, Akifumi*; Ogura, Koichi; Sagisaka, Akito; Orimo, Satoshi; Daito, Izuru*; et al.
Plasma Physics and Controlled Fusion, 57(2), p.025001_1 - 025001_9, 2015/02
Times Cited Count:3 Percentile:12.55(Physics, Fluids & Plasmas)Projection images of a metal mesh produced by directional MeV electron beam together with directional proton beam, emitted simultaneously from a thin foil target irradiated by an ultrashort intense laser. The mesh patterns are projected to each detector by the electron beam and the proton beam originated from tiny virtual sources of  20 micron meter and
20 micron meter and  10 micron meter diameters, respectively. Based on the observed quality and magnification of the projection images, we estimate sizes and locations of the virtual sources for both beams and characterize their directionalities. To carry out physical interpretation of the directional electron beam qualitatively, we perform 2D particle-in-cell simulation which reproduces a directional escaping electron component, together with a non-directional dragged-back electron component, the latter mainly contributes to building a sheath electric field for proton acceleration.
10 micron meter diameters, respectively. Based on the observed quality and magnification of the projection images, we estimate sizes and locations of the virtual sources for both beams and characterize their directionalities. To carry out physical interpretation of the directional electron beam qualitatively, we perform 2D particle-in-cell simulation which reproduces a directional escaping electron component, together with a non-directional dragged-back electron component, the latter mainly contributes to building a sheath electric field for proton acceleration.
 image crystal
image crystalSerizawa, Hiroyuki; Oishi, Yuji*; Kaji, Yoshiyuki; Yamanaka, Shinsuke*
New Research Trends of Fluorite-Based Oxide Materials, p.243 - 262, 2015/01
The image crystal is a shape controllable negative crystal which is sometimes reported by mineralogist. The negative crystals formed in a large single-crystal mass have attracted interest as expensive jewelry because of their mysterious appearance and rarity. Nonetheless, for many scientist, the cavity has been nothing else than a volume defect which occurs in a single crystal by chance. However, the authors found unexpectedly that the image crystal is formed in UO when helium is enclosed in the gas bubble. They also found that the shape of the image crystal changes dramatically with the conditions of helium precipitation, which implies that the shape of the cavity should change depending on the inner pressure. A truncated octahedron-type, an octa-triacontahedron-type and a pentacontahedron-type negative crystal were observed. Recent study clarified that the growth process of the image crystal can be explained by a step free energy model rather than an attachment energy.
when helium is enclosed in the gas bubble. They also found that the shape of the image crystal changes dramatically with the conditions of helium precipitation, which implies that the shape of the cavity should change depending on the inner pressure. A truncated octahedron-type, an octa-triacontahedron-type and a pentacontahedron-type negative crystal were observed. Recent study clarified that the growth process of the image crystal can be explained by a step free energy model rather than an attachment energy.

Tanaka, Kosuke; Tokushima, Kazuyuki*; Kurosaki, Ken*; Oishi, Yuji*; Muta, Hiroaki*; Yamanaka, Shinsuke*
Journal of Nuclear Materials, 443(1-3), p.218 - 221, 2013/11
Times Cited Count:0 Percentile:0.00(Materials Science, Multidisciplinary)Polycrystalline specimens of barium uranate, BaUO , were prepared and several properties such as the thermal expansion coefficient, elastic moduli, thermal conductivity, and Debye temperature were evaluated.
, were prepared and several properties such as the thermal expansion coefficient, elastic moduli, thermal conductivity, and Debye temperature were evaluated.