Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Kawahara, Takahiro; Suda, Shoya; Fujikura, Toshiki; Masai, Seita; Omori, Kanako; Mori, Masakazu; Kurosawa, Tsuyoshi; Ishihara, Keisuke; Hoshi, Akiko; Yokobori, Tomohiko
JAEA-Technology 2023-020, 36 Pages, 2023/12
We have been storing drums containing radioactive waste (radioactive waste packages) at waste storage facilities. We have been managing radioactive waste packages along traditional safety regulations. However, over 40 years has passed from a part of them were brought in pit-type waste storage facility L. Most of them are carbon steel 200 L drums, and surface of them are corroded. For better safety management, we started to take drums out from the pit and inspect them in FY 2019. After each inspection, we repair them or remove the contents of the drum and refill new drums if necessary. In this report, we will introduce the planning, the review of the plan, and the trial operation of this project.
Kurihara, Momo*; Yasutaka, Tetsuo*; Aono, Tatsuo*; Ashikawa, Nobuo*; Ebina, Hiroyuki*; Iijima, Takeshi*; Ishimaru, Kei*; Kanai, Ramon*; Karube, Jinichi*; Konnai, Yae*; et al.
Journal of Radioanalytical and Nuclear Chemistry, 322(2), p.477 - 485, 2019/11
Times Cited Count:4 Percentile:40.00(Chemistry, Analytical)We assessed the repeatability and reproducibility of methods for determining low dissolved radiocesium concentrations in freshwater in Fukushima. Twenty-one laboratories pre-concentrated three of 10 L samples by five different pre-concentration methods (prussian-blue-impregnated filter cartridges, coprecipitation with ammonium phosphomolybdate, evaporation, solid-phase extraction disks, and ion-exchange resin columns), and activity of radiocesium was measured. The z-scores for all of the  Cs results were within
Cs results were within  2, indicating that the methods were accurate. The relative standard deviations (RSDs) indicating the variability in the results from different laboratories were larger than the RSDs indicating the variability in the results from each separate laboratory.
2, indicating that the methods were accurate. The relative standard deviations (RSDs) indicating the variability in the results from different laboratories were larger than the RSDs indicating the variability in the results from each separate laboratory.
Morita, Keisuke; Suzuki, Hideya; Matsumura, Tatsuro; Takahashi, Yuya*; Omori, Takashi*; Kaneko, Masaaki*; Asano, Kazuhito*
Proceedings of International Nuclear Fuel Cycle Conference / Light Water Reactor Fuel Performance Conference (Global/Top Fuel 2019) (USB Flash Drive), p.464 - 468, 2019/09
High level liquid waste (HLLW) contains several radionuclides with half-lives longer than 10 year. For reduce environmental burden of waste disposal, minor actinoids and long-lived fission products will to be partitioned and transmuted. JAEA and Toshiba developed process for recovering Se, Zr, Pd and Cs from HLLW. Solvent extraction for Zr with novel extractant,
year. For reduce environmental burden of waste disposal, minor actinoids and long-lived fission products will to be partitioned and transmuted. JAEA and Toshiba developed process for recovering Se, Zr, Pd and Cs from HLLW. Solvent extraction for Zr with novel extractant,  -didodecyl-2-hydroxyacetoamide (HAA) was detailed. The HAA system showed high selectivity for Zr, as indicated by the extraction order of Zr
-didodecyl-2-hydroxyacetoamide (HAA) was detailed. The HAA system showed high selectivity for Zr, as indicated by the extraction order of Zr  Mo
Mo  Pd
Pd  Ag
Ag  Sb
Sb  Sn
Sn  Lns
Lns  Fe. The extracted species was determined as Zr(HAA)
Fe. The extracted species was determined as Zr(HAA) (NO
(NO )
) (HNO
(HNO )
) . A continuous countercurrent extraction with HAA was applied to a simulated, concentrated HLLW after Pd, Se, and Cs removal, where the quantitative extraction of Zr and Mo was effectively demonstrated.
. A continuous countercurrent extraction with HAA was applied to a simulated, concentrated HLLW after Pd, Se, and Cs removal, where the quantitative extraction of Zr and Mo was effectively demonstrated.
Sasaki, Yuji; Morita, Keisuke; Ito, Keisuke; Suzuki, Shinichi; Shiwaku, Hideaki; Takahashi, Yuya*; Kaneko, Masaaki*; Omori, Takashi*; Asano, Kazuhito*
Proceedings of International Nuclear Fuel Cycle Conference (GLOBAL 2017) (USB Flash Drive), 4 Pages, 2017/09
no abstracts in English
 SO
SO /HNO
/HNO mixed solution
mixed solutionLi, Z.*; Toyoshima, Atsushi; Asai, Masato; Tsukada, Kazuaki; Sato, Tetsuya; Sato, Nozomi; Kikuchi, Takahiro; Nagame, Yuichiro; Sch del, M.; Pershina, V.*; et al.
del, M.; Pershina, V.*; et al.
Radiochimica Acta, 100(3), p.157 - 164, 2012/03
Times Cited Count:14 Percentile:70.88(Chemistry, Inorganic & Nuclear) SR study of impurity effects on the Cu-spin fluctuations in the overdoped regime of La
SR study of impurity effects on the Cu-spin fluctuations in the overdoped regime of La Sr
Sr Cu
Cu Zn
Zn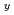 O
O
Risdiana*; Adachi, Tadashi*; Oki, Naoki*; Yairi, Satoshi*; Tanabe, Yoichi*; Omori, Keisuke*; Suzuki, Takao*; Watanabe, Isao*; Koda, Akihiro*; Higemoto, Wataru; et al.
Physica C, 460-462(2), p.874 - 875, 2007/09
Times Cited Count:3 Percentile:17.80(Physics, Applied)Zero-field muon-spin-relaxation measurements have been carried out for La Sr
Sr Cu
Cu Zn
Zn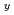 O
O (LSCO)with
(LSCO)with 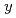 = 0-0.10 in the overdoped regime up to
= 0-0.10 in the overdoped regime up to  = 0.30, in order to investigate whether the dynamical stripe correlations are pinned and stabilized even for the overdoped LSCO or not. It has been found that the Zn-induced slowing down of the Cu-spin fluctuations is weakened with increasing
= 0.30, in order to investigate whether the dynamical stripe correlations are pinned and stabilized even for the overdoped LSCO or not. It has been found that the Zn-induced slowing down of the Cu-spin fluctuations is weakened with increasing  but takes place in the overdoped regime and disappears at
but takes place in the overdoped regime and disappears at  = 0.30. This suggests that the stripe-pinning model holds good in the whole superconducting regime of LSCO and that there is no quantum critical point at
= 0.30. This suggests that the stripe-pinning model holds good in the whole superconducting regime of LSCO and that there is no quantum critical point at 
 0.19.
0.19.
Sugie, Tatsuo; Takeuchi, Masaki; Ishikawa, Masao; Shimada, Takahiko; Katsunuma, Atsushi*; Kitazawa, Daisuke*; Omori, Keisuke*; Itami, Kiyoshi
no journal, ,
no abstracts in English
Ogawa, Hiroaki; Kitazawa, Sin-iti; Sugie, Tatsuo; Katsunuma, Atsushi*; Kitazawa, Daisuke*; Omori, Keisuke*; Itami, Kiyoshi
no journal, ,
no abstracts in English
 solution for understanding chemical species of Db
solution for understanding chemical species of DbAdachi, Sadia*; Sueki, Keisuke*; Toyoshima, Atsushi*; Tsukada, Kazuaki; Haba, Hiromitsu*; Komori, Yukiko*; Yokokita, Takuya*; Mori, Taiki*
no journal, ,
Anion exchange behavior of group-5 elements, Nb, Ta, and their pseudo homologue Pa in HF and HF/HNO solution was investigated to understand chemical properties of Db. Adsorption behavior of Db is clearly different from that of Ta and is similar to those of Nb and Pa. In the present study, anion exchange experiments of Nb, Ta and Pa were carried out with HF/HNO
solution was investigated to understand chemical properties of Db. Adsorption behavior of Db is clearly different from that of Ta and is similar to those of Nb and Pa. In the present study, anion exchange experiments of Nb, Ta and Pa were carried out with HF/HNO aqueous solution as basic experiments for estimating the species of Db fluoride complex. Adsorption behavior was investigated by changing the fluoride ion concentration for each HNO
aqueous solution as basic experiments for estimating the species of Db fluoride complex. Adsorption behavior was investigated by changing the fluoride ion concentration for each HNO concentration. Based on the observed distribution coefficients of these elements, we discuss the formation reaction of the anionic fluoride complex and chemical species of Db.
concentration. Based on the observed distribution coefficients of these elements, we discuss the formation reaction of the anionic fluoride complex and chemical species of Db.
 solution
solutionKato, Mizuho*; Adachi, Sadia*; Toyoshima, Atsushi*; Tsukada, Kazuaki; Asai, Masato; Haba, Hiromitsu*; Yokokita, Takuya*; Komori, Yukiko*; Shigekawa, Yudai*; Sueki, Keisuke*
no journal, ,
A few studies have been so far performed on aqueous chemistry of Db, the group-5 superheavy element, by anion-exchange experiments in HF and HF/HNO mixture solutions. Although the distribution coefficient (Kd) of Db was shown to trend to Ta
mixture solutions. Although the distribution coefficient (Kd) of Db was shown to trend to Ta  Nb
Nb  Db
Db  Pa in the group-5 homologs and pseudo-homolog elements, its chemical species has not been clarified. In this work, for identification of fluoride species of Db, we performed anion-exchange experiments of Nb, Ta, and Db in HF/1.0 M HNO
Pa in the group-5 homologs and pseudo-homolog elements, its chemical species has not been clarified. In this work, for identification of fluoride species of Db, we performed anion-exchange experiments of Nb, Ta, and Db in HF/1.0 M HNO mixture solutions by using an automated rapid chemistry apparatus, ARCA.
mixture solutions by using an automated rapid chemistry apparatus, ARCA.
 SO
SO /HNO
/HNO mixed solution ([H
mixed solution ([H ] = 1.0 M)
] = 1.0 M)Li, Z.; Toyoshima, Atsushi; Asai, Masato; Tsukada, Kazuaki; Sato, Tetsuya; Sato, Nozomi; Kikuchi, Takahiro; Sch del, M.*; Nagame, Yuichiro; Liang, X. H.*; et al.
del, M.*; Nagame, Yuichiro; Liang, X. H.*; et al.
no journal, ,
Cation-exchange behavior of  Rf (T
Rf (T = 78 s) produced in the
= 78 s) produced in the  Cm(
Cm( O, 5n) reaction was studied on a "one-atom-at-a-time" scale in H
O, 5n) reaction was studied on a "one-atom-at-a-time" scale in H SO
SO (0.15-0.69 M)/HNO
(0.15-0.69 M)/HNO mixed solutions ([H
mixed solutions ([H ] = 1.0 M) using an automated ion-exchange separation apparatus coupled with the detection system for alpha-spectroscopy. It was found that adsorption probability (%ads) of
] = 1.0 M) using an automated ion-exchange separation apparatus coupled with the detection system for alpha-spectroscopy. It was found that adsorption probability (%ads) of  Rf on cation-exchange resin decreases with increasing [HSO
Rf on cation-exchange resin decreases with increasing [HSO
 ], showing a successive formation of its sulfate complexes. Rf exhibited much weaker formation of the complexes than the lighter homologues Zr and Hf, which is qualitatively in good agreement with theoretical calculations including relativistic effects.
], showing a successive formation of its sulfate complexes. Rf exhibited much weaker formation of the complexes than the lighter homologues Zr and Hf, which is qualitatively in good agreement with theoretical calculations including relativistic effects.
 rays
raysYamazaki, Takumi; Takada, Chie; Nakamura, Keisuke; Sagawa, Naoki; Hoshi, Katsuya; Nakagawa, Takahiro; Takimoto, Misaki; Tanimura, Yoshihiko*; Takahashi, Fumiaki; Momose, Takumaro; et al.
no journal, ,
no abstracts in English
Sasaki, Yuji; Suzuki, Shinichi; Shiwaku, Hideaki; Ito, Keisuke; Takahashi, Yuya*; Kaneko, Masaaki*; Omori, Takashi*; Asano, Kazuhito*
no journal, ,
no abstracts in English
 SO
SO /HNO
/HNO mixed solution ([H
mixed solution ([H ] = 1.0 M)
] = 1.0 M)Li, Z.; Toyoshima, Atsushi; Asai, Masato; Tsukada, Kazuaki; Sato, Tetsuya; Sato, Nozomi; Sch del, M.*; Nagame, Yuichiro; Liang, X. H.*; Kasamatsu, Yoshitaka*; et al.
del, M.*; Nagame, Yuichiro; Liang, X. H.*; Kasamatsu, Yoshitaka*; et al.
no journal, ,
Cation-exchange behavior of  Rf (T
Rf (T = 78 s) produced in the
= 78 s) produced in the  Cm(
Cm( O,5n) reaction was studied on a "one-atom-at-a-time" scale in 0.15-0.69 M H
O,5n) reaction was studied on a "one-atom-at-a-time" scale in 0.15-0.69 M H SO
SO /HNO
/HNO mixed solutions ([H
mixed solutions ([H ] = 1.0 M) using an automated ion-exchange separation apparatus coupled with a detection system for alpha-spectroscopy (AIDA). It was found that adsorption probabilities (%ads) of Rf on the cation-exchange resin decrease with increasing [HSO
] = 1.0 M) using an automated ion-exchange separation apparatus coupled with a detection system for alpha-spectroscopy (AIDA). It was found that adsorption probabilities (%ads) of Rf on the cation-exchange resin decrease with increasing [HSO
 ], showing successive formation of Rf sulfate complexes. Rf exhibits a weaker complex formation than the lighter homologues Zr and Hf.
], showing successive formation of Rf sulfate complexes. Rf exhibits a weaker complex formation than the lighter homologues Zr and Hf.
Morita, Keisuke; Suzuki, Hideya; Matsumura, Tatsuro; Takahashi, Yuya*; Omori, Takashi*; Kaneko, Masaaki*; Asano, Kazuhito*
no journal, ,
no abstracts in English
Morita, Keisuke; Suzuki, Hideya; Matsumura, Tatsuro; Takahashi, Yuya*; Omori, Takashi*; Kaneko, Masaaki*; Asano, Kazuhito*
no journal, ,
no abstracts in English
Takahashi, Yuya*; Omori, Takashi*; Yamashita, Yu*; Kaneko, Masaaki*; Asano, Kazuhito*; Morita, Keisuke; Suzuki, Hideya*; Matsumura, Tatsuro
no journal, ,
no abstracts in English