Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Fueda, Kazuki*; Komiya, Tatsuki*; Minomo, Kenta*; Horie, Kenji*; Takehara, Mami*; Yamasaki, Shinya*; Shiotsu, Hiroyuki; Onuki, Toshihiko*; Grambow, B.*; Law, G. T. W.*; et al.
Chemosphere, 328, p.138566_1 - 138566_12, 2023/07
Times Cited Count:1 Percentile:52.26(Environmental Sciences)Onuki, Toshihiko*; Ye, J.*; Kato, Tomoaki; Liu, J.; Takano, Masahide; Kozai, Naofumi; Utsunomiya, Satoshi*
Environmental Science; Processes & Impacts, 25(7), p.1204 - 1212, 2023/07
Times Cited Count:0 Percentile:0(Chemistry, Analytical)To elucidate chemical forms of Cs and I in microparticles produced via the Fukushima Daiichi Nuclear Power Plant accident and released into the atmosphere, we analyzed Cs and I in condensed vaporized particles (CVP) produced by melting experiments using nuclear fuel components containing CsI with concrete. CVPs consisted of many round particles containing Cs and I of diameters less than several tens of micrometers. Two kinds of particles were present: one containing large amounts of Cs and I, suggesting the presence of CsI, and the other containing small amounts of Cs and I with large Si contents. Most of CsI from both particles were dissolved in water. On the contrary, some fractions of Cs remained from the latter particles. These results suggest that Cs was incorporated in CVPs along with Si to form water low-soluble CVPs
 sp. DN11 and its potential use for removal of radioiodine from contaminated aquifers
sp. DN11 and its potential use for removal of radioiodine from contaminated aquifersSasamura, Seiya*; Onuki, Toshihiko*; Kozai, Naofumi; Amachi, Seigo*
Frontiers in Microbiology (Internet), 14, p.1162788_1 - 1162788_7, 2023/04
Times Cited Count:2 Percentile:79.73(Microbiology) sp. DN11 previously isolated from gasoline-contaminated groundwater contained a gene cluster involved in bacterial iodate (IO
sp. DN11 previously isolated from gasoline-contaminated groundwater contained a gene cluster involved in bacterial iodate (IO
 ) respiration. This study determined if strain DN11 performed iodate respiration and assessed its potential use to remove and sequester radioactive iodine (
) respiration. This study determined if strain DN11 performed iodate respiration and assessed its potential use to remove and sequester radioactive iodine ( I) from subsurface contaminated aquifers. Strain DN11 grew anaerobically with iodate as the sole electron acceptor. After the growth of strain DN11 on iodate, silver-impregnated zeolite was added to the spent medium to remove iodide from the aqueous phase. In the presence of 200
I) from subsurface contaminated aquifers. Strain DN11 grew anaerobically with iodate as the sole electron acceptor. After the growth of strain DN11 on iodate, silver-impregnated zeolite was added to the spent medium to remove iodide from the aqueous phase. In the presence of 200  M iodate as the electron acceptor, more than 98% of iodine was successfully removed from the aqueous phase. These results suggest that strain DN11 is potentially helpful for bioaugmentation of
M iodate as the electron acceptor, more than 98% of iodine was successfully removed from the aqueous phase. These results suggest that strain DN11 is potentially helpful for bioaugmentation of  I-contaminated subsurface aquifers.
I-contaminated subsurface aquifers.
Liu, J.; Dotsuta, Yuma; Sumita, Takehiro; Kitagaki, Toru; Onuki, Toshihiko; Kozai, Naofumi
Journal of Radioanalytical and Nuclear Chemistry, 331(6), p.2785 - 2794, 2022/06
Times Cited Count:3 Percentile:68.71(Chemistry, Analytical)Remnant nuclear fuel debris in the damaged nuclear reactors at the Fukushima Daiichi Nuclear Power Plant (FDNPP) has contacted the groundwater containing microorganisms for over ten years. Herein, we report the possibility of bacterial alteration of fuel debris. We investigated the physical and chemical changes of fuel debris simulants (FDS) in the powder and pellet forms via exposure to two ubiquitous bacteria, Pseudomonas fluorescens and Bacillus subtilis. In the experiments using FDS composed of the powders of Fe(0), solid solution of CeO and ZrO
and ZrO , and SiO
, and SiO , Ce, Zr, and Si were hardly dissolved, while Fe was dissolved, a fraction of the dissolved Fe was present in the liquid phase as Fe(II) and Fe(III), and the rest was precipitated as the nano-sized particles of iron (hydr)oxides. In the experiment using P. fluorescens and FDS pellet pieces prepared by melting the Fe(0) particles and solid solution of CeO
, Ce, Zr, and Si were hardly dissolved, while Fe was dissolved, a fraction of the dissolved Fe was present in the liquid phase as Fe(II) and Fe(III), and the rest was precipitated as the nano-sized particles of iron (hydr)oxides. In the experiment using P. fluorescens and FDS pellet pieces prepared by melting the Fe(0) particles and solid solution of CeO and ZrO
and ZrO , the bacteria selectively gathered on the Fe(0) particle surface and made corrosion pits. These results suggest that bacteria in groundwater corrode the iron in fuel debris at FDNPP, change fuel debris into porous one, releasing the nano-sized iron (hydr)oxide particles into the water.
, the bacteria selectively gathered on the Fe(0) particle surface and made corrosion pits. These results suggest that bacteria in groundwater corrode the iron in fuel debris at FDNPP, change fuel debris into porous one, releasing the nano-sized iron (hydr)oxide particles into the water.
Kato, Tomoaki; Kozai, Naofumi; Tanaka, Kazuya; Kaplan, D. I.*; Utsunomiya, Satoshi*; Onuki, Toshihiko
Journal of Nuclear Science and Technology, 59(5), p.580 - 589, 2022/05
Times Cited Count:4 Percentile:56.94(Nuclear Science & Technology)This study reports the effect of fulvic acids, which is a natural organic substance generally contained in groundwater, on the oxidation states of radioactive iodine anions (iodide and iodate). Iodide and iodate are contained in the contaminated water of the Fukushima Daiichi Nuclear Power Plant and supposed to be removed by activated carbon (AC) via adsorption. When fulvic acids does not exist in the experimental system, the adsorption of iodide on AC was less than that of iodate and their oxidation states after the adsorption were not changed. When fulvic acids existed, a fraction of the adsorbed iodate was reduced to iodide. This result indicates that the reduction of the adsorbed iodate progresses during the storage of the spent AC.
 C control rods in Fukushima Daiichi nuclear reactors during meltdown; B-Li isotopic signatures in cesium-rich microparticles
C control rods in Fukushima Daiichi nuclear reactors during meltdown; B-Li isotopic signatures in cesium-rich microparticlesFueda, Kazuki*; Takami, Ryu*; Minomo, Kenta*; Morooka, Kazuya*; Horie, Kenji*; Takehara, Mami*; Yamasaki, Shinya*; Saito, Takumi*; Shiotsu, Hiroyuki; Onuki, Toshihiko*; et al.
Journal of Hazardous Materials, 428, p.128214_1 - 128214_10, 2022/04
Times Cited Count:6 Percentile:68.71(Engineering, Environmental)Tanaka, Kazuya; Tani, Yukinori*; Kozai, Naofumi; Onuki, Toshihiko
Journal of Radioanalytical and Nuclear Chemistry, 331(2), p.1109 - 1114, 2022/02
Times Cited Count:0 Percentile:0.01(Chemistry, Analytical)We investigated the sorption of Pu(IV) on biogenic Mn oxide produced by Mn(II)-oxidizing fungus. The sorption of Pu(IV) on biogenic Mn oxide was similar to that of U(VI) and different from that of Th(IV), possibly due to oxidation of Pu(IV) to Pu(VI). When Pu(IV) was sorbed on hyphae only, it was desorbed into the solution phase over time. Pu(IV) could be solubilized by complexation with organic ligands secreted by fungal cells. In particular, Pu(IV) desorption was observed under circumneutral pH conditions.
Grambow, B.; Nitta, Ayako; Shibata, Atsuhiro; Koma, Yoshikazu; Utsunomiya, Satoshi*; Takami, Ryu*; Fueda, Kazuki*; Onuki, Toshihiko*; Jegou, C.*; Laffolley, H.*; et al.
Journal of Nuclear Science and Technology, 59(1), p.1 - 24, 2022/01
Times Cited Count:15 Percentile:71.66(Nuclear Science & Technology)Kozai, Naofumi; Sato, Junya; Osugi, Takeshi; Shimoyama, Iwao; Sekine, Yurina; Sakamoto, Fuminori; Onuki, Toshihiko
Journal of Hazardous Materials, 416, p.125965_1 - 125965_9, 2021/08
Times Cited Count:24 Percentile:85.21(Engineering, Environmental)Deng, G.*; Ma, T.*; Tanaka, Kazuya; Onuki, Toshihiko*; Qiu, X.*; Yu, Q.*
Geochimica et Cosmochimica Acta, 286, p.143 - 158, 2020/10
Times Cited Count:4 Percentile:29.05(Geochemistry & Geophysics)In this study, Ce(III) adsorption on Mn(IV) oxide was investigated in the presence of dextran, one of polysaccharides. As a result, Ce(IV) on Mn(IV) oxide was solubilized by the complexation with dextran.
Guo, B.*; Xiong, Y.*; Chen, W.*; Saslow, S. A.*; Kozai, Naofumi; Onuki, Toshihiko*; Dabo, I.*; Sasaki, Keiko*
Journal of Hazardous Materials, 389, p.121880_1 - 121880_11, 2020/05
Times Cited Count:43 Percentile:90.61(Engineering, Environmental)Onuki, Toshihiko*; Ozaki, Takuo*; Kozai, Naofumi; Utsunomiya, Satoshi*
Behavior of Radionuclides in the Environment I; Function of Particles in Aquatic System, p.67 - 92, 2020/00
It has been experimentally revealed that microorganisms transform radionuclides. For example, cells of microorganisms such as bacteria and yeast accumulate actinides on the surface. Phosphate ions released from microorganism cells precipitate actinides as phosphates. This chapter discusses the role of environmental microorganisms on migration of actinides in the groundwater around Lake Karachai where a lot of radioactive wastes from nuclear facilities of the Soviet Union.

 on Ni-Zn layered hydroxide salt and its application to removal of ReO
on Ni-Zn layered hydroxide salt and its application to removal of ReO
 as a surrogate of TcO
as a surrogate of TcO

Tanaka, Kazuya; Kozai, Naofumi; Yamasaki, Shinya*; Onuki, Toshihiko; Kaplan, D. I.*; Grambow, B.
Applied Clay Science, 182, p.105282_1 - 105282_8, 2019/12
Times Cited Count:16 Percentile:67.61(Chemistry, Physical)In this study, Ni-Zn layered hydroxide salt (LHS) was used for adsorption experiments of ReO
 , as a surrogate of TcO
, as a surrogate of TcO
 , in aqueous solutions with various initial Re and sodium salt concentrations. The maximum adsorption amount of Re was estimated at 127.7 mg/g (6.86
, in aqueous solutions with various initial Re and sodium salt concentrations. The maximum adsorption amount of Re was estimated at 127.7 mg/g (6.86  10
10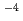 eq/g) by fitting adsorption isotherm of ReO
eq/g) by fitting adsorption isotherm of ReO
 to Langmuir plot. The adsorption of ReO
to Langmuir plot. The adsorption of ReO
 at neutral pH was a reversible process by anion exchange, and decreased with increasing Cl
at neutral pH was a reversible process by anion exchange, and decreased with increasing Cl , NO
, NO
 and SO
and SO
 in solution. EXAFS analysis indicated that ReO
in solution. EXAFS analysis indicated that ReO
 was adsorbed as an outer-sphere complex on Ni-Zn LHS. The Ni-Zn LHS is a more robust adsorbent for ReO
was adsorbed as an outer-sphere complex on Ni-Zn LHS. The Ni-Zn LHS is a more robust adsorbent for ReO
 than the Mg-Al LDH in terms of solution pH and tolerance to competing anions, and may be an effective alternative to the traditional and more limited method of removing aqueous TcO
than the Mg-Al LDH in terms of solution pH and tolerance to competing anions, and may be an effective alternative to the traditional and more limited method of removing aqueous TcO
 by reductive precipitation.
by reductive precipitation.
 , Zn
, Zn +, and Co
+, and Co
Kato, Tomoaki*; Yu, Q.*; Tanaka, Kazuya; Kozai, Naofumi; Saito, Takumi*; Onuki, Toshihiko
Journal of Environmental Sciences, 86, p.78 - 86, 2019/12
Times Cited Count:2 Percentile:8.42(Environmental Sciences)This paper investigated the fate of the dissolved permanganate in aqueous solution after contact with bacterial cells and metal accumulation during precipitation of Mn oxides. When Mn(VII) was contacted with bacterial cells, cells were damaged and Mn(VII) was reduced by cells to lower valence and precipitated as Mn oxides (biomass Mn oxides). When Co ions were present, Co was incorporated into Mn oxides as Co
ions were present, Co was incorporated into Mn oxides as Co . These results suggest that Mn(VII) can be used to remove metal ions when introduced to wastewater as disinfectant.
. These results suggest that Mn(VII) can be used to remove metal ions when introduced to wastewater as disinfectant.
Onuki, Toshihiko*; Satou, Yukihiko; Utsunomiya, Satoshi*
Journal of Nuclear Science and Technology, 56(9-10), p.790 - 800, 2019/09
Times Cited Count:9 Percentile:63.25(Nuclear Science & Technology)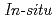 investigation of radioactive Cs mobility around litter zone in contaminated forest using spent mushroom substrata
investigation of radioactive Cs mobility around litter zone in contaminated forest using spent mushroom substrataOnuki, Toshihiko*; Sakamoto, Fuminori; Kozai, Naofumi; Yamasaki, Shinya*; Sasaki, Yoshito; Niizato, Tadafumi
Journal of Nuclear Science and Technology, 56(9-10), p.814 - 821, 2019/09
Times Cited Count:2 Percentile:21.58(Nuclear Science & Technology)We used the spent mushroom substrata (SMSs) which are a kind of by-product after growing edible mushrooms for the 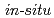 investigation of radioactive Cs mobility in litter zone in a forest of Fukushima prefecture, Japan. The powder SMS was filled in a plastic net bag of 0.35
investigation of radioactive Cs mobility in litter zone in a forest of Fukushima prefecture, Japan. The powder SMS was filled in a plastic net bag of 0.35 0.55 m, then was placed in a forest for
0.55 m, then was placed in a forest for  6 months under three kinds of different conditions without treatment (No treatment), covered with wooden box (With box), and with zeolite placed on upper position of ground surface (With zeolite). We determined the ratio of radioactivity (TF) in the SMS to that of the soil and litter beneath the SMS bags. TFs of "No treatment" and of "With zeolite" were determined between
6 months under three kinds of different conditions without treatment (No treatment), covered with wooden box (With box), and with zeolite placed on upper position of ground surface (With zeolite). We determined the ratio of radioactivity (TF) in the SMS to that of the soil and litter beneath the SMS bags. TFs of "No treatment" and of "With zeolite" were determined between  0.01 and
0.01 and  0.05 for 6 months. On the other hand, TFs of "With box" were lower by one order at 2 and 4 months than those of "No treatment" and of "With zeolite", and nearly the same values as TFs of "No treatment" and "With zeolite" at 6 months. These results clearly indicate that radioactive Cs accumulates in SMS mainly by throughfall. In addition, for a period of several months, fungi contribute to the accumulation of radioactive Cs in the litter zone, even though radioactive Cs was tightly associated with the soil.
0.05 for 6 months. On the other hand, TFs of "With box" were lower by one order at 2 and 4 months than those of "No treatment" and of "With zeolite", and nearly the same values as TFs of "No treatment" and "With zeolite" at 6 months. These results clearly indicate that radioactive Cs accumulates in SMS mainly by throughfall. In addition, for a period of several months, fungi contribute to the accumulation of radioactive Cs in the litter zone, even though radioactive Cs was tightly associated with the soil.
Onuki, Toshihiko; Sakamoto, Fuminori; Kozai, Naofumi; Namba, Kenji*; Neda, Hitoshi*; Sasaki, Yoshito; Niizato, Tadafumi; Watanabe, Naoko*; Kozaki, Tamotsu*
Environmental Science; Processes & Impacts, 21(7), p.1164 - 1173, 2019/07
Times Cited Count:10 Percentile:44.69(Chemistry, Analytical)The fate of radioactive Cs deposited after the Fukushima nuclear power plant accident and its associated radiological impacts are largely dependent on its mobility from surface soils to forest ecosystems. We measured the accumulation of radioactive Cs in the fruit bodies of wild fungi in the forest at Iidate, Fukushima, Japan. The transfer factors (TFs) of radioactive Cs from soil to the fruit bodies of wild fungi were between 10 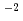 to 10
to 10 , a range similar to those reported for the fruit bodies collected in Europe after the Chernobyl accident and in parts of Japan contaminated by nuclear bomb test fallout. Comparison of the TFs of the wild mushroom and that of the fungal hyphae of 704 stock strains grown on agar medium containing nutrients and radioactive Cs showed that the TFs of wild mushroom were lower. TF was less than 0.1 after addition of the minerals zeolite, vermiculite, phlogopite, smectite, or illite of 1% weight to the agar medium. These results indicate that the presence of minerals decrease Cs uptake by fungi grown in the agar medium.
, a range similar to those reported for the fruit bodies collected in Europe after the Chernobyl accident and in parts of Japan contaminated by nuclear bomb test fallout. Comparison of the TFs of the wild mushroom and that of the fungal hyphae of 704 stock strains grown on agar medium containing nutrients and radioactive Cs showed that the TFs of wild mushroom were lower. TF was less than 0.1 after addition of the minerals zeolite, vermiculite, phlogopite, smectite, or illite of 1% weight to the agar medium. These results indicate that the presence of minerals decrease Cs uptake by fungi grown in the agar medium.
Liu, J.; Dotsuta, Yuma; Kitagaki, Toru; Kozai, Naofumi; Yamaji, Keiko*; Onuki, Toshihiko
Proceedings of International Topical Workshop on Fukushima Decommissioning Research (FDR 2019) (Internet), 2 Pages, 2019/05
To decommission the Fukushima Daiichi Nuclear Power Plant (FDNPP), it is necessary to estimate the current status of fuel debris and predicate the possible change under various condition. Some microorganisms may enter the plant due to the seawater injection after accident and future defueling activity. In this study, microbial influence on fuel debris under aerobic condition was experimentally investigated. By culturing some bacteria in the presence of simulant fuel debris in liquid medium, the microbial degradation of fuel debris was observed.
Tanaka, Kazuya; Kozai, Naofumi; Onuki, Toshihiko; Grambow, B.
Journal of Porous Materials, 26(2), p.505 - 511, 2019/04
Times Cited Count:8 Percentile:31.21(Chemistry, Applied)In this study, we utilized X-ray absorption fine structure spectroscopy to clarify the coordination structure of Re in Mg-Al LDH as a surrogate of Tc. Adsorption experiments of ReO
 on calcined and uncalcined Mg-Al LDHs were conducted in aqueous solutions with different concentrations of NaCl, NaNO
on calcined and uncalcined Mg-Al LDHs were conducted in aqueous solutions with different concentrations of NaCl, NaNO , and Na
, and Na SO
SO . Calcined Mg-Al LDH showed much higher adsorption than uncalcined one. The adsorption of ReO
. Calcined Mg-Al LDH showed much higher adsorption than uncalcined one. The adsorption of ReO
 was reversible, and decreased with increasing concentration of competing anions like Cl
was reversible, and decreased with increasing concentration of competing anions like Cl , NO
, NO
 , or SO
, or SO
 . Analysis of Re L
. Analysis of Re L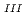 -edge extended X-ray absorption fine structure indicated that ReO
-edge extended X-ray absorption fine structure indicated that ReO
 was adsorbed as an outer-sphere complex on Mg-Al LDH. The observed Re adsorption-desorption behavior, which was sensitive to the presence of competing anions, was consistent with the formation of outer sphere-complex.
was adsorbed as an outer-sphere complex on Mg-Al LDH. The observed Re adsorption-desorption behavior, which was sensitive to the presence of competing anions, was consistent with the formation of outer sphere-complex.
Yu, Q.*; Tanaka, Kazuya; Kozai, Naofumi; Sakamoto, Fuminori; Tani, Yukinori*; Onuki, Toshihiko
ACS Earth and Space Chemistry (Internet), 2(8), p.797 - 810, 2018/08
Times Cited Count:14 Percentile:56.1(Chemistry, Multidisciplinary)Most of Mn oxides are biogenic and known to adsorb cesium (Cs) on the surface. This study investigated structural transformation of biogenic birnessite by accommodating commonly occurring natural heavy metals (Zn, Ni) during the formation of Mn oxides and the influence of those metals on the adsorption behavior of Cs on Mn oxides. It was found that the presence of heavy metals during bio-oxidation of Mn(II), followed by exposure to a low pH aqueous solution, increased the number of available layer vacancies, which consequently increased the adsorption capacity of Cs in the final product birnessite.