Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Omasa, Yoshinori*; Takagi, Shigeyuki*; Toshima, Kento*; Yokoyama, Kaito*; Endo, Wataru*; Orimo, Shinichi*; Saito, Hiroyuki*; Yamada, Takeshi*; Kawakita, Yukinobu; Ikeda, Kazutaka*; et al.
Physical Review Research (Internet), 4(3), p.033215_1 - 033215_9, 2022/09
Fukutani, Katsuyuki; Yoshinobu, Jun*; Yamauchi, Miho*; Shima, T.*; Orimo, Shinichi*
Catalysis Letters, 152, p.1583 - 1597, 2022/06
Times Cited Count:9 Percentile:49.02(Chemistry, Physical)Saito, Hiroyuki*; Machida, Akihiko*; Hattori, Takanori; Sano, Asami; Funakoshi, Kenichi*; Sato, Toyoto*; Orimo, Shinichi*; Aoki, Katsutoshi*
Physica B; Condensed Matter, 587, p.412153_1 - 412153_6, 2020/06
Times Cited Count:2 Percentile:12.9(Physics, Condensed Matter)The site occupancy of deuterium (D) atoms in face-centered-cubic nickel (fcc Ni) was measured along a cooling path from 1073 to 300 K at an initial pressure of 3.36 GPa via in situ neutron powder diffraction. Deuterium atoms predominantly occupy the octahedral (O) sites and slightly occupy the tetrahedral (T) sites of the fcc metal lattice. The O-site occupancy increases from 0.4 to 0.85 as the temperature is lowered from 1073 to 300 K. Meanwhile, the T-site occupancy remains c.a. 0.02. The temperature-independent behavior of the T-site occupancy is unusual, and its process is not yet understood. From the linear relation between the expanded lattice volume and D content, a D-induced volume expansion of 2.09(13) 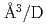 atom was obtained. This value is in agreement with the values of 2.14-2.2
atom was obtained. This value is in agreement with the values of 2.14-2.2 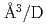 atom previously reported for Ni and Ni
atom previously reported for Ni and Ni Fe
Fe alloy.
alloy.
Saito, Hiroyuki*; Machida, Akihiko*; Iizuka, Riko*; Hattori, Takanori; Sano, Asami; Funakoshi, Kenichi*; Sato, Toyoto*; Orimo, Shinichi*; Aoki, Katsutoshi*
Scientific Reports (Internet), 10, p.9934_1 - 9934_8, 2020/06
Times Cited Count:3 Percentile:15.07(Multidisciplinary Sciences)Neutron powder diffraction profiles were collected for iron deuteride (FeDx) while the temperature decreased from 1023 to 300 K for a pressure range of 4-6 GPa. The  ' deuteride with a double hexagonal close-packed (dhcp) structure, which coexisted with other stable or metastable deutrides at each temperature and pressure condition, formed solid solutions with a composition of FeD
' deuteride with a double hexagonal close-packed (dhcp) structure, which coexisted with other stable or metastable deutrides at each temperature and pressure condition, formed solid solutions with a composition of FeD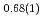 at 673 K and 6.1 GPa and FeD
at 673 K and 6.1 GPa and FeD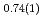 at 603 K and 4.8 GPa. Upon stepwise cooling to 300 K, the D-content x increased to a stoichiometric value of 1.0 to form monodeuteride FeD
at 603 K and 4.8 GPa. Upon stepwise cooling to 300 K, the D-content x increased to a stoichiometric value of 1.0 to form monodeuteride FeD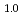 . In the dhcp FeD
. In the dhcp FeD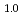 at 300 K and 4.2 GPa, dissolved D atoms fully occupied the octahedral interstitial sites, slightly displaced from the octahedral centers in the dhcp metal lattice, and the dhcp sequence of close-packed Fe planes contained hcp-stacking faults at 12%. Magnetic moments with 2.11
at 300 K and 4.2 GPa, dissolved D atoms fully occupied the octahedral interstitial sites, slightly displaced from the octahedral centers in the dhcp metal lattice, and the dhcp sequence of close-packed Fe planes contained hcp-stacking faults at 12%. Magnetic moments with 2.11  0.06 B/Fe-atom aligned ferromagnetically in parallel on the Fe planes.
0.06 B/Fe-atom aligned ferromagnetically in parallel on the Fe planes.
Machida, Akihiko*; Saito, Hiroyuki*; Hattori, Takanori; Sano, Asami; Funakoshi, Kenichi*; Sato, Toyoto*; Orimo, Shinichi*; Aoki, Katsutoshi*
Scientific Reports (Internet), 9(1), p.12290_1 - 12290_9, 2019/08
Times Cited Count:25 Percentile:83.96(Multidisciplinary Sciences)Hexagonal close-packed iron hydride, hcp FeHx, is absent from the conventional phase diagram of the Fe-H system, although hcp metallic Fe exists stably over extensive temperature ( ) and pressure (
) and pressure ( ) conditions, including those corresponding to the Earth's inner core.
) conditions, including those corresponding to the Earth's inner core. 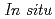 X-ray and neutron diffraction measurements at temperatures ranging from 298 to 1073 K and H pressures ranging from 4 to 7 GPa revealed that the hcp hydride was formed for FeH
X-ray and neutron diffraction measurements at temperatures ranging from 298 to 1073 K and H pressures ranging from 4 to 7 GPa revealed that the hcp hydride was formed for FeH compositions when
compositions when 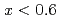 . Hydrogen atoms occupied the octahedral interstitial sites of the host metal lattice both partially and randomly. The hcp hydride exhibited a H-induced volume expansion of 2.48(5)
. Hydrogen atoms occupied the octahedral interstitial sites of the host metal lattice both partially and randomly. The hcp hydride exhibited a H-induced volume expansion of 2.48(5) 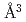 /H-atom, which was larger than that of the face-centered cubic (fcc) hydride. The hcp hydride showed an increase in
/H-atom, which was larger than that of the face-centered cubic (fcc) hydride. The hcp hydride showed an increase in  with
with  , whereas the fcc hydride showed a corresponding decrease. The present study provides guidance for further investigations of the Fe-H system over an extensive
, whereas the fcc hydride showed a corresponding decrease. The present study provides guidance for further investigations of the Fe-H system over an extensive  -
- -
- region.
region.
Takagi, Shigeyuki*; Iijima, Yuki*; Sato, Toyoto*; Saito, Hiroyuki; Ikeda, Kazutaka*; Otomo, Toshiya*; Miwa, Kazutoshi*; Ikeshoji, Tamio*; Aoki, Katsutoshi*; Orimo, Shinichi*
Angewandte Chemie; International Edition, 54(19), p.5650 - 5653, 2015/05
Times Cited Count:32 Percentile:68.44(Chemistry, Multidisciplinary)Machida, Akihiko; Saito, Hiroyuki; Sugimoto, Hidehiko*; Hattori, Takanori; Sano, Asami; Endo, Naruki*; Katayama, Yoshinori; Iizuka, Riko*; Sato, Toyoto*; Matsuo, Motoaki*; et al.
Nature Communications (Internet), 5, p.5063_1 - 5063_6, 2014/09
Times Cited Count:55 Percentile:86.02(Multidisciplinary Sciences)Iron hydride FeH , is thermodynamically stable only at high hydrogen pressure of several GPa. To investigate the hydrogenation process and hydrogen state in iron hydride, it is necessary to carry out the in-situ measurement under high pressure and high temperature. In this study, we performed the in-situ neutron diffraction measurement of Fe-D system using the high pressure neutron diffractometer PLANET in the MLF, J-PARC, and determined the deuterium occupying sites and occupancies in fcc-FeD
, is thermodynamically stable only at high hydrogen pressure of several GPa. To investigate the hydrogenation process and hydrogen state in iron hydride, it is necessary to carry out the in-situ measurement under high pressure and high temperature. In this study, we performed the in-situ neutron diffraction measurement of Fe-D system using the high pressure neutron diffractometer PLANET in the MLF, J-PARC, and determined the deuterium occupying sites and occupancies in fcc-FeD . We found the minor occupation of tetrahedral sites under high pressure and high temperature. We considered the mechanism of the minor occupation based on the Quantum-mechanical calculation.
. We found the minor occupation of tetrahedral sites under high pressure and high temperature. We considered the mechanism of the minor occupation based on the Quantum-mechanical calculation.
 FeH
FeH ; Iron-containing complex hydride with high gravimetric hydrogen density
; Iron-containing complex hydride with high gravimetric hydrogen densitySaito, Hiroyuki; Takagi, Shigeyuki*; Matsuo, Motoaki*; Iijima, Yuki*; Endo, Naruki*; Aoki, Katsutoshi*; Orimo, Shinichi*
APL Materials (Internet), 2(7), p.076103_1 - 076103_7, 2014/07
Times Cited Count:25 Percentile:70.44(Nanoscience & Nanotechnology) synchrotron radiation X-ray diffraction measurement
synchrotron radiation X-ray diffraction measurementSaito, Hiroyuki; Takagi, Shigeyuki*; Aoki, Katsutoshi; Orimo, Shinichi*
Hoshako, 27(1), p.10 - 19, 2014/01
no abstracts in English
 on dehydrogenation of magnesium borohydride Mg(BH
on dehydrogenation of magnesium borohydride Mg(BH )
)
Li, H.-W.*; Matsumura, Daiju; Nishihata, Yasuo; Akiba, Etsuo*; Orimo, Shinichi*
Nihon Kinzoku Gakkai-Shi, 77(12), p.627 - 630, 2013/12
Times Cited Count:4 Percentile:27.48(Metallurgy & Metallurgical Engineering)no abstracts in English
 CuH
CuH ; A New class of interstitial aluminum-based alloy hydride
; A New class of interstitial aluminum-based alloy hydrideSaito, Hiroyuki; Takagi, Shigeyuki*; Endo, Naruki; Machida, Akihiko; Aoki, Katsutoshi; Orimo, Shinichi*; Katayama, Yoshinori
APL Materials (Internet), 1(3), p.032113_1 - 032113_7, 2013/09
Times Cited Count:15 Percentile:54.06(Nanoscience & Nanotechnology) RuH
RuH ; Prospect for formation of [FeH
; Prospect for formation of [FeH ]
] anion
anionTakagi, Shigeyuki*; Ikeshoji, Tamio*; Matsuo, Motoaki*; Sato, Toyoto*; Saito, Hiroyuki; Aoki, Katsutoshi; Orimo, Shinichi*
Applied Physics Letters, 103(11), p.113903_1 - 113903_4, 2013/09
Times Cited Count:9 Percentile:37.98(Physics, Applied) ;
;  synchrotron radiation X-ray diffraction study
synchrotron radiation X-ray diffraction studySato, Ryutaro*; Saito, Hiroyuki; Endo, Naruki; Takagi, Shigeyuki*; Matsuo, Motoaki*; Aoki, Katsutoshi; Orimo, Shinichi*
Applied Physics Letters, 102(9), p.091901_1 - 091901_4, 2013/03
Times Cited Count:29 Percentile:73.44(Physics, Applied) and its synthesis; Mechanism for formation of metallic perovskite
and its synthesis; Mechanism for formation of metallic perovskiteTakagi, Shigeyuki*; Saito, Hiroyuki; Endo, Naruki; Sato, Ryutaro*; Ikeshoji, Tamio*; Matsuo, Motoaki*; Miwa, Kazutoshi*; Aoki, Katsutoshi; Orimo, Shinichi*
Physical Review B, 87(12), p.125134_1 - 125134_6, 2013/03
Times Cited Count:14 Percentile:53.53(Materials Science, Multidisciplinary) ; Adjustment of imbalanced charge by additional Li as an electron donor
; Adjustment of imbalanced charge by additional Li as an electron donorMatsuo, Motoaki*; Saito, Hiroyuki; Machida, Akihiko; Sato, Ryutaro*; Takagi, Shigeyuki*; Miwa, Kazutoshi*; Watanuki, Tetsu; Katayama, Yoshinori; Aoki, Katsutoshi; Orimo, Shinichi*
RSC Advances (Internet), 3(4), p.1013 - 1016, 2013/01
Times Cited Count:19 Percentile:53.62(Chemistry, Multidisciplinary) H
H on formation kinetics and X-ray absorption fine structure analyses
on formation kinetics and X-ray absorption fine structure analysesMatsuo, Motoaki*; Matsumura, Daiju; Nishihata, Yasuo; Li, G.*; Hiyama, Nao*; Semboshi, Satoshi*; Orimo, Shinichi*
Applied Physics Letters, 100(4), p.044101_1 - 044101_3, 2012/01
Times Cited Count:4 Percentile:18.19(Physics, Applied)Matsumura, Daiju; Oyama, Takahiro*; Okajima, Yuka*; Nishihata, Yasuo; Li, H.-W.*; Orimo, Shinichi*
Materials Transactions, 52(4), p.635 - 640, 2011/04
Times Cited Count:13 Percentile:54.56(Materials Science, Multidisciplinary)Local structure of Ti-based additives in Mg(BH )
) was studied by X-ray absorption fine structure spectroscopy with conventional and dispersive mode in order to understand the correlation between the structure of the additives and dehydrogenation property of Mg(BH
was studied by X-ray absorption fine structure spectroscopy with conventional and dispersive mode in order to understand the correlation between the structure of the additives and dehydrogenation property of Mg(BH )
) . Simultaneous measurement of the dehydrogenation curve and the X-ray absorption spectroscopy by the dispersive optics revealed that a part of TiCl
. Simultaneous measurement of the dehydrogenation curve and the X-ray absorption spectroscopy by the dispersive optics revealed that a part of TiCl additive is converted to Ti
additive is converted to Ti Mg
Mg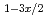 (BH
(BH )
) (
( = 0
= 0 0.67) just after ball milling mixture and then promptly resolved to TiB
0.67) just after ball milling mixture and then promptly resolved to TiB at 100-150
at 100-150 C with the first dehydrogenation peak. TiO
C with the first dehydrogenation peak. TiO additive is slowly converted to lower oxidation state in wide temperature range around the second dehydrogenation peak at 350
additive is slowly converted to lower oxidation state in wide temperature range around the second dehydrogenation peak at 350 C where the main dehydrogenation reaction of Mg(BH
C where the main dehydrogenation reaction of Mg(BH )
) occurs.
occurs.
Ikeda, Kazuki*; Orimo, Shinichi*; Saito, Hiroyuki; Machida, Akihiko; Katayama, Yoshinori; Aoki, Katsutoshi
Suiso Seizo, Kyuzo, Chozo Zairyo To Anzenka, p.317 - 327, 2010/08
AlH is a metal hydride with a large gravimetric and volumetric hydrogen content (10.1 wt.% and 149kgH
is a metal hydride with a large gravimetric and volumetric hydrogen content (10.1 wt.% and 149kgH /m
/m , respectively), and therefore it is promising as a hydrogen storage material. The high capacity and low desorption temperature (80
, respectively), and therefore it is promising as a hydrogen storage material. The high capacity and low desorption temperature (80 150
150 C) have attracted much attention. In situ X-ray diffraction measurements for the Al-H binary system at high pressures and temperatures were performed to investigate the hydrogenation of aluminum metal immersed in hydrogen fluid as well as the phase stability of the aluminum hydride formed.
C) have attracted much attention. In situ X-ray diffraction measurements for the Al-H binary system at high pressures and temperatures were performed to investigate the hydrogenation of aluminum metal immersed in hydrogen fluid as well as the phase stability of the aluminum hydride formed.
Suzuki, Masayuki; Kiriyama, Hiromitsu; Daito, Izuru; Okada, Hajime; Nakai, Yoshiki; Orimo, Satoshi; Sato, Masatoshi*; Tamaoki, Yoshinori*; Yoshii, Takehiro*; Maeda, Junya*; et al.
Applied Physics B, 97(2), p.379 - 382, 2009/10
Times Cited Count:7 Percentile:37.56(Optics)We report the highest energy broadband laser pulses at a center wavelength of 1030 nm based on optical parametric chirped-pulse amplification (OPCPA). We have demonstrated amplification of 1030 nm femtosecond laser pulses from a broadband Yb oscillator to over 6.5 mJ with a total gain of greater than 10 achieved in a single pass through only 56 mm of gain material at a 10 Hz repetition rate. The amplified spectral bandwidth of 10.8 nm affords recompression to a 230 fs pulse duration following amplification. As an alternative to the regenerative amplifier (RA) this system is one of the more promising candidates for realizing compact, high intensity, direct diode pumped, high repetition rate femtosecond Yb:YAG chirped-pulse amplification (CPA) in laser systems.
achieved in a single pass through only 56 mm of gain material at a 10 Hz repetition rate. The amplified spectral bandwidth of 10.8 nm affords recompression to a 230 fs pulse duration following amplification. As an alternative to the regenerative amplifier (RA) this system is one of the more promising candidates for realizing compact, high intensity, direct diode pumped, high repetition rate femtosecond Yb:YAG chirped-pulse amplification (CPA) in laser systems.
Yogo, Akifumi; Sato, Katsutoshi; Nishikino, Masaharu; Mori, Michiaki; Teshima, Teruki*; Numasaki, Hodaka*; Murakami, Masao*; Demizu, Yusuke*; Akagi, Takashi*; Nagayama, Shinichi*; et al.
Applied Physics Letters, 94(18), p.181502_1 - 181502_3, 2009/05
Times Cited Count:110 Percentile:94.75(Physics, Applied)