Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
 O
OKondo, Yosuke*; Achouri, N. L.*; Al Falou, H.*; Atar, L.*; Aumann, T.*; Baba, Hidetada*; Boretzky, K.*; Caesar, C.*; Calvet, D.*; Chae, H.*; et al.
Nature, 620(7976), p.965 - 970, 2023/08
Times Cited Count:29 Percentile:95.28(Multidisciplinary Sciences)no abstracts in English
 Ne at the transition into the island of inversion; Detailed structure study of
Ne at the transition into the island of inversion; Detailed structure study of  Ne
NeWang, H.*; Yasuda, Masahiro*; Kondo, Yosuke*; Nakamura, Takashi*; Tostevin, J. A.*; Ogata, Kazuyuki*; Otsuka, Takaharu*; Poves, A.*; Shimizu, Noritaka*; Yoshida, Kazuki; et al.
Physics Letters B, 843, p.138038_1 - 138038_9, 2023/08
Times Cited Count:4 Percentile:67.28(Astronomy & Astrophysics)Detailed  -ray spectroscopy of the exotic neon isotope
-ray spectroscopy of the exotic neon isotope  Ne has been performed using the one-neutron removal reaction from
Ne has been performed using the one-neutron removal reaction from 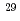 Ne. Based on an analysis of parallel momentum distributions, a level scheme with spin-parity assignments has been constructed for
Ne. Based on an analysis of parallel momentum distributions, a level scheme with spin-parity assignments has been constructed for 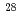 Ne and the negative-parity states are identified for the first time. The measured partial cross sections and momentum distributions reveal a significant intruder p-wave strength providing evidence of the breakdown of the N = 20 and N = 28 shell gaps. Only a weak, possible f-wave strength was observed to bound final states. Large-scale shell-model calculations with different effective interactions do not reproduce the large p-wave and small f-wave strength observed experimentally, indicating an ongoing challenge for a complete theoretical description of the transition into the island of inversion along the Ne isotopic chain.
Ne and the negative-parity states are identified for the first time. The measured partial cross sections and momentum distributions reveal a significant intruder p-wave strength providing evidence of the breakdown of the N = 20 and N = 28 shell gaps. Only a weak, possible f-wave strength was observed to bound final states. Large-scale shell-model calculations with different effective interactions do not reproduce the large p-wave and small f-wave strength observed experimentally, indicating an ongoing challenge for a complete theoretical description of the transition into the island of inversion along the Ne isotopic chain.
Iwamoto, Osamu; Iwamoto, Nobuyuki; Kunieda, Satoshi; Minato, Futoshi; Nakayama, Shinsuke; Abe, Yutaka*; Tsubakihara, Kosuke*; Okumura, Shin*; Ishizuka, Chikako*; Yoshida, Tadashi*; et al.
Journal of Nuclear Science and Technology, 60(1), p.1 - 60, 2023/01
Times Cited Count:213 Percentile:99.99(Nuclear Science & Technology)Murase, Kiyoka*; Kataoka, Ryuho*; Nishiyama, Takanori*; Nishimura, Koji*; Hashimoto, Taishi*; Tanaka, Yoshimasa*; Kadokura, Akira*; Tomikawa, Yoshihiro*; Tsutsumi, Masaki*; Ogawa, Yasunobu*; et al.
Journal of Space Weather and Space Climate (Internet), 12, p.18_1 - 18_16, 2022/06
Times Cited Count:3 Percentile:28.94(Astronomy & Astrophysics)We identified two energetic electron precipitation (EEP) events during the growth phase of moderate substorms and estimated the mesospheric ionization rate for an EEP event for which the most comprehensive dataset from ground-based and space-born instruments was available. The mesospheric ionization signature reached below 70 km altitude and continued for ~15 min until the substorm onset, as observed by the PANSY radar and imaging riometer at Syowa Station in the Antarctic region. We also used energetic electron flux observed by the Arase and POES 15 satellites as the input for the air-shower simulation code PHITS to quantitatively estimate the mesospheric ionization rate. Combining the cutting-edge observations and simulations, we shed new light on the space weather impact of the EEP events during geomagnetically quiet times, which is important to understand the possible link between the space environment and climate.
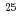 F from
F from  O
O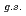 ?
?Tang, T. L.*; Uesaka, Tomohiro*; Kawase, Shoichiro; Beaumel, D.*; Dozono, Masanori*; Fujii, Toshihiko*; Fukuda, Naoki*; Fukunaga, Taku*; Galindo-Uribarri, A.*; Hwang, S. H.*; et al.
Physical Review Letters, 124(21), p.212502_1 - 212502_6, 2020/05
Times Cited Count:19 Percentile:72.96(Physics, Multidisciplinary)The structure of a neutron-rich 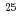 F nucleus is investigated by a quasifree (
F nucleus is investigated by a quasifree (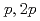 ) knockout reaction. The sum of spectroscopic factors of
) knockout reaction. The sum of spectroscopic factors of 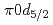 orbital is found to be 1.0
orbital is found to be 1.0  0.3. The result shows that the
0.3. The result shows that the  O core of
O core of 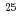 F nucleus significantly differs from a free
F nucleus significantly differs from a free  O nucleus, and the core consists of
O nucleus, and the core consists of  35%
35%  O
O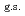 , and
, and  65% excited
65% excited  O. The result shows that the
O. The result shows that the  O core of
O core of 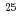 F nucleus significantly differs from a free
F nucleus significantly differs from a free  O nucleus. The result may infer that the addition of the
O nucleus. The result may infer that the addition of the 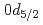 proton considerably changes the neutron structure in
proton considerably changes the neutron structure in 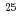 F from that in
F from that in  O, which could be a possible mechanism responsible for the oxygen dripline anomaly.
O, which could be a possible mechanism responsible for the oxygen dripline anomaly.
Uchiyama, Shoichiro*; Ishihara, Ryo*; Umeno, Daisuke*; Saito, Kyoichi*; Yamada, Shinsuke*; Hirota, Hideyuki*; Asai, Shiho
Journal of Chemical Engineering of Japan, 46(6), p.414 - 419, 2013/06
Times Cited Count:7 Percentile:24.57(Engineering, Chemical)Ito, Takachika*; Suzuki, Shoichi*; Kanaji, Sachiko*; Shiraishi, Hiroshi*; Ota, Shoichiro*; Arima, Kazuhiko*; Tanaka, Go*; Tamada, Taro; Honjo, Eijiro*; Garcia, K. C.*; et al.
Journal of Biological Chemistry, 284(36), p.24289 - 24296, 2009/09
Times Cited Count:24 Percentile:45.35(Biochemistry & Molecular Biology)Both IL-4 and IL-13 can bind to the shared receptor composed of the IL-4 receptor  chain and the IL-13 receptor
chain and the IL-13 receptor  -1 chain (IL-13R
-1 chain (IL-13R 1); however, the assembly mechanisms of these ligands to the receptor is different, enabling the principal functions of these ligands to be different. We have previously shown that the N-terminal Ig-like domain in IL-13R
1); however, the assembly mechanisms of these ligands to the receptor is different, enabling the principal functions of these ligands to be different. We have previously shown that the N-terminal Ig-like domain in IL-13R 1, called the D1 domain, is the specific and critical binding unit for IL-13. However, it has still remained obscure which the amino acid has specific binding capacity to IL-13 and why the D1 domain acts as the binding site for IL-13, but not IL-4. To address these questions, in this study, we performed the mutational analyses for the D1 domain, combining the structural data to identify the amino acids critical for binding to IL-13. Mutations of Lys76, Lys77, or Ile78 in c' strand in which the crystal structure showed interact with IL-13 and those of Trp65 and Ala79 adjacent to the interacting site, resulted in significant impairment of IL-13 binding, demonstrating that these amino acids generate the binding site. Furthermore, mutations of Val35, Leu38, or Val42 at N-terminal
1, called the D1 domain, is the specific and critical binding unit for IL-13. However, it has still remained obscure which the amino acid has specific binding capacity to IL-13 and why the D1 domain acts as the binding site for IL-13, but not IL-4. To address these questions, in this study, we performed the mutational analyses for the D1 domain, combining the structural data to identify the amino acids critical for binding to IL-13. Mutations of Lys76, Lys77, or Ile78 in c' strand in which the crystal structure showed interact with IL-13 and those of Trp65 and Ala79 adjacent to the interacting site, resulted in significant impairment of IL-13 binding, demonstrating that these amino acids generate the binding site. Furthermore, mutations of Val35, Leu38, or Val42 at N-terminal  -strand also resulted in loss of IL-13 binding, probably from decrease structural stability. None of the mutations employed here affected IL-4 binding. These results demonstrate that the hydrophobic patch composed of Lys76, Lys77, and Ile78 is the IL-13 recognition site and solidify our understanding that the differential requirements of the D1 domain in IL-13R
-strand also resulted in loss of IL-13 binding, probably from decrease structural stability. None of the mutations employed here affected IL-4 binding. These results demonstrate that the hydrophobic patch composed of Lys76, Lys77, and Ile78 is the IL-13 recognition site and solidify our understanding that the differential requirements of the D1 domain in IL-13R 1 allows the shared receptor to respond differentially to IL-4 and IL-13.
1 allows the shared receptor to respond differentially to IL-4 and IL-13.
 2 chain (IL-13R
2 chain (IL-13R 2)
2)Matsumoto, Fumiko; Tamada, Taro; Honjo, Eijiro*; Ota, Shoichiro*; Izuhara, Kenji*; Kuroki, Ryota
no journal, ,
The allergic disease has dramatically increased in recent years. Interleukin-13 (IL-13) is a key cytokine critical to the development of T-cell-mediated humoral immune responses which are associated with allergy and asthma. IL-13 possesses two types of receptor: the heterodimer, composed of IL-13R 1 and IL-4R
1 and IL-4R , transducing the IL-13 signals; and the IL-13R
, transducing the IL-13 signals; and the IL-13R 2, acting as a nonsignaling "decoy" receptor. Although the extracellular region of IL-13R
2, acting as a nonsignaling "decoy" receptor. Although the extracellular region of IL-13R 1 and IL-13R
1 and IL-13R 2 are composed of a common structure of the class 1 cytokine receptor superfamily and they have similar amino acid sequence, the affinity of IL-13R
2 are composed of a common structure of the class 1 cytokine receptor superfamily and they have similar amino acid sequence, the affinity of IL-13R 2 with IL-13 is more than ten-fold higher than that of IL-13R
2 with IL-13 is more than ten-fold higher than that of IL-13R 1. In order to investigate the reason for this higher ligand affinity to IL-13R
1. In order to investigate the reason for this higher ligand affinity to IL-13R 2, extra cellular region of IL-13R
2, extra cellular region of IL-13R 2 was expressed by silkworm-baculovirus expression system after fusing the DNA cording the extracellular region of human IL-13R
2 was expressed by silkworm-baculovirus expression system after fusing the DNA cording the extracellular region of human IL-13R 2 with the Fc derived from mouse IgG2a. The expressed fusion protein IL-13R
2 with the Fc derived from mouse IgG2a. The expressed fusion protein IL-13R 2-Fc was purified by a protein G column, and the affinity to IL-13 was confirmed by gel filtration followed by light scattering analysis. After Fc region was removed by thrombin digestion, the resulting extra cellular region of IL-13R
2-Fc was purified by a protein G column, and the affinity to IL-13 was confirmed by gel filtration followed by light scattering analysis. After Fc region was removed by thrombin digestion, the resulting extra cellular region of IL-13R 2 receptor was confirmed to retain strong affinity to IL-13 with 1:1 ratio.
2 receptor was confirmed to retain strong affinity to IL-13 with 1:1 ratio.
 2 chain
2 chainMatsumoto, Fumiko; Hatanaka, Takaaki*; Tamada, Taro; Honjo, Eijiro*; Ota, Shoichiro*; Ito, Yuji*; Izuhara, Kenji*; Kuroki, Ryota
no journal, ,
Interleukin-13 (IL-13) is a key cytokine critical to the development of T-cell-mediated humoral immune responses which are associated with allergy and asthma. IL-13 possesses two types of receptor: the heterodimer, composed of IL-13R 1 and IL-4R
1 and IL-4R , transducing the IL-13 signals; and the IL-13R
, transducing the IL-13 signals; and the IL-13R 2, it has high affinity rather than IL-13R
2, it has high affinity rather than IL-13R 1 against IL-13, acting as a nonsignaling "decoy" receptor. In order to investigate the inhibition mechanism of IL-13 signal by IL-13R
1 against IL-13, acting as a nonsignaling "decoy" receptor. In order to investigate the inhibition mechanism of IL-13 signal by IL-13R 2, extra cellular region of IL-13R
2, extra cellular region of IL-13R 2 was expressed by silkworm-baculovirus expression system after fusing the DNA cording the extracellular region of human IL-13R
2 was expressed by silkworm-baculovirus expression system after fusing the DNA cording the extracellular region of human IL-13R 2 with the Fc derived from mouse IgG2a. The expressed fusion protein (IL-13R
2 with the Fc derived from mouse IgG2a. The expressed fusion protein (IL-13R 2)2-Fc was purified by a protein A column followed by an ion exchange column. The affinities of the ligand complexes, IL-13/IL-13R
2)2-Fc was purified by a protein A column followed by an ion exchange column. The affinities of the ligand complexes, IL-13/IL-13R 1 and IL-13/IL-13R
1 and IL-13/IL-13R 2 to (IL-4R
2 to (IL-4R )2-Fc were investigated to explore the inactivation mechanism of the IL-13R
)2-Fc were investigated to explore the inactivation mechanism of the IL-13R 1 signal with IL-13R
1 signal with IL-13R 2 by surface plasmon resonance analysis. IL-13/IL-13R
2 by surface plasmon resonance analysis. IL-13/IL-13R 1 complex has an affinity (KD=1.20
1 complex has an affinity (KD=1.20 10
10 M) with (IL-4Ra)2-Fc, whereas the IL-13/IL-13R
M) with (IL-4Ra)2-Fc, whereas the IL-13/IL-13R 2 complex had no affinity with (IL-4R
2 complex had no affinity with (IL-4R )2-Fc. This observation suggests that IL-13R
)2-Fc. This observation suggests that IL-13R 2 does not inactivate the IL-13R
2 does not inactivate the IL-13R 1 through formation of a ternary complex with IL-13 and IL-4R
1 through formation of a ternary complex with IL-13 and IL-4R , but removes the IL-13 with its higher affinity to IL-13 without forming a ternary complex.
, but removes the IL-13 with its higher affinity to IL-13 without forming a ternary complex.