Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Minato, Kazuo; Konashi, Kenji*; Fujii, Toshiyuki*; Uehara, Akihiro*; Nagasaki, Shinya*; Otori, Norikazu*; Tokunaga, Yo; Kambe, Shinsaku
IOP Conference Series; Materials Science and Engineering, 9, p.012018_1 - 012018_7, 2010/05
Times Cited Count:0 Percentile:1.02(Chemistry, Inorganic & Nuclear)Basic research in actinide chemistry and physics is indispensable to maintain sustainable development of innovative nuclear technology. Actinides, especially minor actinides of americium and curium, need to be handled in special facilities with containment and radiation shields. To promote and facilitate the actinide research, close cooperation with the facilities and sharing of technical and scientific information must be very important and effective. A three-year-program "Basic actinide chemistry and physics research in close cooperation with hot laboratories", ACTILAB, was started to form the bases of sustainable development of innovative nuclear technology. In this program, researches on actinide solid-state physics, solution chemistry and solid-liquid interface chemistry are made using four main facilities in Japan in close cooperation with each other, where basic experiments with transuranium elements can be made. The  O-NMR measurements were performed on (Pu
O-NMR measurements were performed on (Pu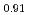 Am
Am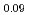 )O
)O to study the electronic state and the chemical behaviour of Am and Cm ions in electrolyte solutions was studied by distribution experiments.
to study the electronic state and the chemical behaviour of Am and Cm ions in electrolyte solutions was studied by distribution experiments.
Otori, Norikazu; Ueno, Fumiyoshi; Furukawa, Tomohiro*
Denki Kagaku Oyobi Kogyo Butsuri Kagaku, 73(8), p.597 - 599, 2005/08
Raman spectra have been obtained for peroxide ion in Na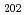 up to 950 K and in molten NaOH up to 900 K. Drastic changes were observed in the spectra around 780 K, which was able to be attributed to the phase transition between beta and alfa forms. The spectra of peroxide ions in molten NaOH was obtained at 610 K just above the eutectic point and the peak can be observed up to 900 K for the melt containing 20 mol% Na
up to 950 K and in molten NaOH up to 900 K. Drastic changes were observed in the spectra around 780 K, which was able to be attributed to the phase transition between beta and alfa forms. The spectra of peroxide ions in molten NaOH was obtained at 610 K just above the eutectic point and the peak can be observed up to 900 K for the melt containing 20 mol% Na 2O
2O . This shows evidently that peroxide ions is able to be present in molten NaOH. An analysis of the peak position suggests the analogous local structure around peroxide ions with that in the crystal.
. This shows evidently that peroxide ions is able to be present in molten NaOH. An analysis of the peak position suggests the analogous local structure around peroxide ions with that in the crystal.
 Raman spectroscopic observation of corrosion reaction of Fe with Na
Raman spectroscopic observation of corrosion reaction of Fe with Na O
O up to 833 K
up to 833 KOtori, Norikazu; Furukawa, Tomohiro*; Ueno, Fumiyoshi
Denki Kagaku Oyobi Kogyo Butsuri Kagaku, 73(8), p.675 - 679, 2005/08
Raman spectra have been obtained for alfa- and beta-NaFeO , Na
, Na FeO
FeO , Na
, Na Fe
Fe O
O , Na
, Na FeO
FeO , and Na
, and Na FeO
FeO from room temperature up to 723 K in a state of powder under an inert atmosphere. The comparison of the spectra showed good applicability of Raman spectroscopy to the in situ identification between these sodium iron double oxides. On the basis of this result, we have investigated corrosion reaction on surface of steel with Na
from room temperature up to 723 K in a state of powder under an inert atmosphere. The comparison of the spectra showed good applicability of Raman spectroscopy to the in situ identification between these sodium iron double oxides. On the basis of this result, we have investigated corrosion reaction on surface of steel with Na O
O powder using in situ Raman spectroscopy. The obtained spectra showed corrosion reaction occurs under 723 K and the corrosion product was identified as Na5FeO4. A reaction mechanism for the corrosion based on the above results was presented that the system of Fe + Na2O2 produces corrosive melt under 723 K so that it spreads over the surface and the corrosion products distribute homogeneously on the surface. The corrosion reaction in homogeneous powder mixture of Fe and Na
powder using in situ Raman spectroscopy. The obtained spectra showed corrosion reaction occurs under 723 K and the corrosion product was identified as Na5FeO4. A reaction mechanism for the corrosion based on the above results was presented that the system of Fe + Na2O2 produces corrosive melt under 723 K so that it spreads over the surface and the corrosion products distribute homogeneously on the surface. The corrosion reaction in homogeneous powder mixture of Fe and Na O
O has also been investigated using Raman spectroscopy with the help of XRD and DTA methods. The corrosion products were identified as the double oxides whose compositions coorespond to the stoichiometric ratio of Na to Fe in the starting materials, while the products from the surface reaction of steel with Na
has also been investigated using Raman spectroscopy with the help of XRD and DTA methods. The corrosion products were identified as the double oxides whose compositions coorespond to the stoichiometric ratio of Na to Fe in the starting materials, while the products from the surface reaction of steel with Na O
O tend to have a spcified composition of Na
tend to have a spcified composition of Na FeO
FeO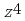 . The differnce can reasonably be explained using the mechanism presented above. It was found from the DTA measurements that Na
. The differnce can reasonably be explained using the mechanism presented above. It was found from the DTA measurements that Na O
O has particularly strong corrosivity for iron, in contrast to Na
has particularly strong corrosivity for iron, in contrast to Na O and NaOH.
O and NaOH.
Otori, Norikazu; Furukawa, Tomohiro
JNC TN9400 2005-005, 33 Pages, 2005/03
We have investigated corrosion reaction on surface of steel with Na O
O powder using in situ Raman spectroscopy. The obtained spectra showed corrosion reaction occurs under 833 K and the corrosion product was identified as Na
powder using in situ Raman spectroscopy. The obtained spectra showed corrosion reaction occurs under 833 K and the corrosion product was identified as Na FeO
FeO . A reaction mechanism for the corrosion based on the above results was presented that the system of Fe + Na
. A reaction mechanism for the corrosion based on the above results was presented that the system of Fe + Na O
O produces corrosive melt under 833 K so that it spreads over the surface and the corrosion products distribute homogeneously on the surface. The corrosion reaction in homogeneous powder mixture of Fe and Na
produces corrosive melt under 833 K so that it spreads over the surface and the corrosion products distribute homogeneously on the surface. The corrosion reaction in homogeneous powder mixture of Fe and Na O
O has also been investigated using Raman spectroscopy with the help of XRD and DTA methods. The corrosion products were identified as the double oxides whose compositions correspond to the stoichiometric ratio of Na to Fe in the starting materials, while the products from the surface reaction of steel with Na
has also been investigated using Raman spectroscopy with the help of XRD and DTA methods. The corrosion products were identified as the double oxides whose compositions correspond to the stoichiometric ratio of Na to Fe in the starting materials, while the products from the surface reaction of steel with Na O
O tend to have a specified composition of Na
tend to have a specified composition of Na FeO
FeO . The difference can reasonably be explained using the mechanism presented above. It was found from the DTA measurements that Na
. The difference can reasonably be explained using the mechanism presented above. It was found from the DTA measurements that Na O
O has particularly strong corrosivity for iron, in contrast to Na
has particularly strong corrosivity for iron, in contrast to Na O and NaOH.
O and NaOH.
Otori, Norikazu; Furukawa, Tomohiro*
Abstract No.PP67, p.166, 166 Pages, 2004/06
Focusing on the cover layer materials (as the Radon Barrier Materials), which could have the effect to restrain the radon from scattering into the air and the effect of the radiation shielding, we produced the radon barrier materials with crude bentonite on an experimental basis, using the rotary type comprehensive unit for grinding and mixing, through which we carried out the evaluation of the characteristics thereof.
Otori, Norikazu; Ueno, Fumiyoshi
JNC TN9400 2002-012, 79 Pages, 2002/04
When sodium is burned at high temperature in the atmosphere, it reacts simultaneously with H 0 in the atmosphere so that it can produce high temperature melt of sodium hydroxide as a solvent. If this melt includes peroxide ion (O
0 in the atmosphere so that it can produce high temperature melt of sodium hydroxide as a solvent. If this melt includes peroxide ion (O
 ), it will be a considerably active and corrosive for iron so that several sodium iron double oxides will be produced as corrosion products after the reaction with steel structures. The present study was carried out in order to investigate the ability of presence of peroxide ion in sodium hydroxide solvent at high temperature and that of identification of the several corrosion products using laser Raman spectroscopy. The measurement system with ultraviolet laser was developed simultaneously in the present work to improve the ability of the measurement at high temperature. As results from the measurements, the possibility of the presence of peroxide ion was shown up to 873K in sodium peroxide and 823K in the melt of sodium hydroxide mixed with sodium peroxide. As for superoxide ion, the possibility of the presence was showed up to 873K in potassium superoxide and up to 773K in the melt of sodium hydroxide mixed with potassium superoxide. As a result from consideration, it was concluded that superoxide ion will not change into peroxide ion in sodium hydroxide solvent at high temperature. On the basis of the above results, it was concluded that the possibility of presence of peroxide ion was shown as a corrosive chemical species in the molten sodium hydroxide at high temperature and this gave an evidence for the reaction mechanism of "melting salt type corrosion". As for sodium iron double oxides, Raman spectra for
), it will be a considerably active and corrosive for iron so that several sodium iron double oxides will be produced as corrosion products after the reaction with steel structures. The present study was carried out in order to investigate the ability of presence of peroxide ion in sodium hydroxide solvent at high temperature and that of identification of the several corrosion products using laser Raman spectroscopy. The measurement system with ultraviolet laser was developed simultaneously in the present work to improve the ability of the measurement at high temperature. As results from the measurements, the possibility of the presence of peroxide ion was shown up to 873K in sodium peroxide and 823K in the melt of sodium hydroxide mixed with sodium peroxide. As for superoxide ion, the possibility of the presence was showed up to 873K in potassium superoxide and up to 773K in the melt of sodium hydroxide mixed with potassium superoxide. As a result from consideration, it was concluded that superoxide ion will not change into peroxide ion in sodium hydroxide solvent at high temperature. On the basis of the above results, it was concluded that the possibility of presence of peroxide ion was shown as a corrosive chemical species in the molten sodium hydroxide at high temperature and this gave an evidence for the reaction mechanism of "melting salt type corrosion". As for sodium iron double oxides, Raman spectra for  -NaFeO
-NaFeO ,
,  -NaFeO
-NaFeO , Na
, Na FeO
FeO , Na
, Na FeO
FeO , and Na
, and Na FeO
FeO has been successfully measured up to 573K. The obtained spectra show the usefulness of Raman spectroscopy as a chemical identification method for these sodium iron double oxides at high temperature up to 573K.
has been successfully measured up to 573K. The obtained spectra show the usefulness of Raman spectroscopy as a chemical identification method for these sodium iron double oxides at high temperature up to 573K.
Ueno, Fumiyoshi; Otori, Norikazu
JNC TN9400 2002-007, 23 Pages, 2002/03
High-temperature sodium on exposure to the air produces a corrosive melt including Na O, Na
O, Na O
O and NaOH. If the melt contacts with steel, corrosion reaction occurs and several sodium-iron complex oxides (Na
and NaOH. If the melt contacts with steel, corrosion reaction occurs and several sodium-iron complex oxides (Na FeO
FeO , Na
, Na FeOt, Na
FeOt, Na FeO
FeO , etc.) produce depending on temperature, basicity and oxygen potential. In this study, as an approach to clarily the crystallne structure of the complex oxides, laser Raman measurements of the complex oxides were carried out to obtain fundamental Raman spectra of synthesized pure Na
, etc.) produce depending on temperature, basicity and oxygen potential. In this study, as an approach to clarily the crystallne structure of the complex oxides, laser Raman measurements of the complex oxides were carried out to obtain fundamental Raman spectra of synthesized pure Na FeO
FeO , Na
, Na FeO
FeO and Na
and Na FeO
FeO in room temperature. Additionally, the results were compared with commercially available sodium-iron compounds and distinguishable patterns in the typical spectra of each complex oxide were examined. As the results, the distinguishable patterns were found for each oxide. Moreover, irreversible changes of the spectra under exposure to the higher laser power were found out so that appropriate laser power was recognized for Raman analysis of the complex oxides, in addition, the same irreversible spectra change was also found under high temperature up to 573K in Ar gas atmosphere as under exposure to the higher laser power.
in room temperature. Additionally, the results were compared with commercially available sodium-iron compounds and distinguishable patterns in the typical spectra of each complex oxide were examined. As the results, the distinguishable patterns were found for each oxide. Moreover, irreversible changes of the spectra under exposure to the higher laser power were found out so that appropriate laser power was recognized for Raman analysis of the complex oxides, in addition, the same irreversible spectra change was also found under high temperature up to 573K in Ar gas atmosphere as under exposure to the higher laser power.
Otori, Norikazu; Ueno, Fumiyoshi
EUCHEM2002 Molten Salts Conference, 22 Pages, 2002/00
None
 O
O by MD simulation
by MD simulationSuzuki, Yoshihiro*; Takase, Keiichi*; Akiyama, Isao*; Suzuya, Kentaro; Umesaki, Norimasa*; Otori, Norikazu*
Materials Transactions, 42(11), p.2242 - 2246, 2001/11
Times Cited Count:7 Percentile:47.39(Materials Science, Multidisciplinary)We have performed the molecular dynamics (MD) simulations for vitreous P O
O using isotropic pair potentials composed only of coulombic and repulsive interaction. The obtained P-O pair distribution function reproduced the two peaks expected from the results of neutron diffraction experiments, in the nearest-neighbor P-O correlation. The neutron-weighted real-space correlation function were also in semi-quantitative agreement with that from the experimental results. The distribution of coordination number for O around P and P around O showed that most P atoms form tetrahedral PO
using isotropic pair potentials composed only of coulombic and repulsive interaction. The obtained P-O pair distribution function reproduced the two peaks expected from the results of neutron diffraction experiments, in the nearest-neighbor P-O correlation. The neutron-weighted real-space correlation function were also in semi-quantitative agreement with that from the experimental results. The distribution of coordination number for O around P and P around O showed that most P atoms form tetrahedral PO units in the glass and three-fifths of O atoms are bridging oxygen, O
units in the glass and three-fifths of O atoms are bridging oxygen, O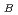 , and the others are terminal one, O
, and the others are terminal one, O . The pair distribution functions for P-O
. The pair distribution functions for P-O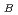 and P-O
and P-O clarified the PO
clarified the PO units have three long P-O
units have three long P-O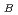 bonds and one short P-O
bonds and one short P-O bond. We have concluded that the short-range structure for vitreous P
bond. We have concluded that the short-range structure for vitreous P O
O agrees well with the picture derived from many experiments.
agrees well with the picture derived from many experiments.
Ueno, Fumiyoshi; Otori, Norikazu
JNC TN9400 2000-097, 55 Pages, 2000/09
If high temperature sodium is burned in humid air, its compound will become high temperature melt of sodium hydroxide as a solvent. If this melt includes peroxide ion, it will be a considerably active aggressive and corrosive for steels. This study was carried out in order to investigate the ability of presence of peroxide ion in sodium hydroxide solvent at high temperature. Laser Raman Spectroscopy was employed to analyze the Raman spectra of sodium peroxide and mixed specimen of sodium hydroxide with sodium peroxide. These reagents were heated up to 1073K under Ar gas atmosphere in the specially made vesse1. Detectability and stability of peroxide ion at high temperature were investigated. Also we investigated about superoxide ion by using sodium peroxide and potassium superoxide. Superoxide ion was induded in sodium peroxide as an impurity. As the result, the stability of peroxide ion in high temperature melt was showed up to 873K for sodium peroxide and 823K for sodium hydroxide mixed with sodium peroxide. And as the results for superoxide ion, its stability was showed as up to 873K for unmixed potassium superoxide and up to 773K for sodium hydroxide mixed one. Additionally, it was considered that superoxide ion will not change into peroxide ion in sodium hydroxide solvent at high temperature.
Otori, Norikazu; *
JNC TJ9400 2001-002, 56 Pages, 1999/03
High temperature Raman spectroscopy and X-ray diffraction technique were applied to a series of sodium oxides, iron oxides and sodium and iron double oxide from ambient to high temperature for the purpose of examining the usefulness of these methods for the in situ chemical analysis in corrosive environments at elevated temperature on the basis of the necessity for direct observations of chemical reactions between burning sodium and iron-base materials. As a result of Raman scattering measurements at backscattering geometly using a furnace containing a crucible cell, an intense peak assignable to a lattice vibration was observed up to 673 K for Na O, that to stretching mode of O
O, that to stretching mode of O
 ion up to 873K, and that to stretching mode of O
ion up to 873K, and that to stretching mode of O
 ion up to 773K for Na
ion up to 773K for Na O
O . The temperature range in which the method is applicable to the detection of these materials was discussed. As for Na
. The temperature range in which the method is applicable to the detection of these materials was discussed. As for Na O, the structural change may limit the upper temperature, whereas, as to Na
O, the structural change may limit the upper temperature, whereas, as to Na O
O , the color change or the decomposition may limit it. The color change suggests the change of electronic structure such as generation of color center. The spectra for
, the color change or the decomposition may limit it. The color change suggests the change of electronic structure such as generation of color center. The spectra for  -NaFeO
-NaFeO were clearly observed up to 873K, while the background caused by radiation at high temperature considerably increased. For higher temperature measurements, the present method will be hopeful, using an ultraviolet light source in order to reduce the background. As a result of X-ray diffraction measurements, all the obtained patterns agreed well with JCPDS cards data, which shows that the method is useful for the chemical analysis of these materials at high temperature up to 1073K. The obtained spectra and difiraction patterns are collected in this report in order to be available on the chemical analysis of more complex mixtures.
were clearly observed up to 873K, while the background caused by radiation at high temperature considerably increased. For higher temperature measurements, the present method will be hopeful, using an ultraviolet light source in order to reduce the background. As a result of X-ray diffraction measurements, all the obtained patterns agreed well with JCPDS cards data, which shows that the method is useful for the chemical analysis of these materials at high temperature up to 1073K. The obtained spectra and difiraction patterns are collected in this report in order to be available on the chemical analysis of more complex mixtures.
Otori, Norikazu*; *
PNC TJ9642 98-001, 24 Pages, 1998/03
Raman spectroscopy was applied to sodium oxides and sodium and iron double oxide from ambient to high temperature for the purpose of establishing it as an in-situ method of chemical analysis at elevated temperature, based on the necessity for direct observations of chemical reactions between burning sodium and iron-base materials. X-ray diffraction method was also applied to the iron double oxide from ambient to high temperature in order to determine its phase directly. The several findings were obtained as follows. As a result of Raman scattering measurements of Na O, an intense peak, which could be assigned to a lattice vibration in the antifluorite structure, was observed around 200 cm
O, an intense peak, which could be assigned to a lattice vibration in the antifluorite structure, was observed around 200 cm up to 823 K. The color of commercially available Na
up to 823 K. The color of commercially available Na O
O was light yellow but changed to black above 573 K. The Raman scattering was, however, observed for the O
was light yellow but changed to black above 573 K. The Raman scattering was, however, observed for the O
 stretching mode from ambient to 773 K. Based on Raman scattering and X-ray diffraction measurements of a sintered mixture of 2Na
stretching mode from ambient to 773 K. Based on Raman scattering and X-ray diffraction measurements of a sintered mixture of 2Na CO
CO and Fe
and Fe O
O 3, the chemical changes might be proposed from ambient to high temperature for example as follows: [Na
3, the chemical changes might be proposed from ambient to high temperature for example as follows: [Na CO
CO
 Na
Na O+CO
O+CO ] and [NaFeO
] and [NaFeO +Na
+Na O
O  Na
Na FeO
FeO .]
.]
Myochin, Munetaka; Kofuji, Hirohide; Yamana, Hajimu*; Shirai, Osamu*; Yamamura, Tsutomu*; Umesaki, Norimasa*; Matsuura, Haruaki*; Kajinami, Akihiko*; Iwadate, Yasuhiko*; Otori, Norikazu*; et al.
no journal, ,
no abstracts in English
Minato, Kazuo; Konashi, Kenji*; Fujii, Toshiyuki*; Nagasaki, Shinya*; Otori, Norikazu*
no journal, ,
no abstracts in English
Minato, Kazuo; Konashi, Kenji*; Fujii, Toshiyuki*; Uehara, Akihiro*; Nagasaki, Shinya*; Otori, Norikazu*; Akabori, Mitsuo; Takano, Masahide; Hayashi, Hirokazu; Tokunaga, Yo; et al.
no journal, ,
Basic research in actinide chemistry and physics is indispensable to maintain sustainable development of innovative nuclear technology. Actinides, especially minor actinides (MA) of americium and curium, need to be handled in special facilities with containment and radiation shields. A three-year-program Basic actinide chemistry and physics research in close cooperation with hot laboratories, ACTILAB, was started to form the basis of sustainable development of innovative nuclear technology. For the basic understanding of the properties of MA-bearing fuels, XAFS (X-ray Absorption Fine Structure) measurements on AmO and
and  O-NMR (Nuclear Magnetic Resonance) measurements on (Pu
O-NMR (Nuclear Magnetic Resonance) measurements on (Pu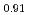 Am
Am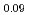 )O
)O were carried out. Curium nitride was synthesized by carbothermic reduction method and the lattice parameters and thermal expansion of CmN were measured by high temperature X-ray diffractometry after purification of aged curium oxide.
were carried out. Curium nitride was synthesized by carbothermic reduction method and the lattice parameters and thermal expansion of CmN were measured by high temperature X-ray diffractometry after purification of aged curium oxide.