Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Ouchi, Kazuki; Ueno, Katsuhiro; Watanabe, Masayuki
Scientific Reports (Internet), 15, p.18515_1 - 18515_7, 2025/05
Times Cited Count:0We first demonstrate a nonaqueous rechargeable battery using uranium and iron as active materials. This uranium-iron battery achieves an open-circuit voltage of approximately 1.3 V, exhibits stable cycling performance, and delivers a good Coulombic efficiency of 86 2%. These characteristics suggest a promising avenue for utilizing depleted uranium in innovative applications.
2%. These characteristics suggest a promising avenue for utilizing depleted uranium in innovative applications.
Yomogida, Takumi; Ouchi, Kazuki; Morii, Shiori; Oka, Toshitaka; Kitatsuji, Yoshihiro; Koma, Yoshikazu; Konno, Katsuhiro*
Scientific Reports (Internet), 14, p.14945_1 - 14945_11, 2024/06
Times Cited Count:1 Percentile:36.75(Multidisciplinary Sciences)Particles containing alpha ( ) nuclides were identified from sediment in stagnant water in the Unit 3 reactor building of the Fukushima Daiichi Nuclear Power Station (FDiNPS). We analyzed different concentrations of alpha nuclides samples collected at two sampling sites, torus room and Main steam isolation valve (MSIV) room. Most of the
) nuclides were identified from sediment in stagnant water in the Unit 3 reactor building of the Fukushima Daiichi Nuclear Power Station (FDiNPS). We analyzed different concentrations of alpha nuclides samples collected at two sampling sites, torus room and Main steam isolation valve (MSIV) room. Most of the  -nuclides in the stagnant water samples of the torus room and the MSIV room were present in particle fractions larger than 10
-nuclides in the stagnant water samples of the torus room and the MSIV room were present in particle fractions larger than 10  m. We detected uranium-bearing particles in
m. We detected uranium-bearing particles in  m-size by scanning electron microscopy-energy dispersive X-Ray (SEM-EDX) observation. Other short lived
m-size by scanning electron microscopy-energy dispersive X-Ray (SEM-EDX) observation. Other short lived  -nuclides were detected by alpha track detection. The
-nuclides were detected by alpha track detection. The  -nuclide-containing particles with several tens to several hundred
-nuclide-containing particles with several tens to several hundred  m in size were mainly comprised iron (Fe) by SEM-EDX analysis. This study clarifies that the morphologies of U and other
m in size were mainly comprised iron (Fe) by SEM-EDX analysis. This study clarifies that the morphologies of U and other  -nuclides in the sediment of stagnant water in the FDiNPS's Unit 3 reactor building.
-nuclides in the sediment of stagnant water in the FDiNPS's Unit 3 reactor building.
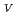 O
O
 /U
/U O
O
 redox equilibrium in [emim]Tf
redox equilibrium in [emim]Tf N-based liquid electrolytes towards uranium redox-flow battery
N-based liquid electrolytes towards uranium redox-flow batteryTakao, Koichiro*; Ouchi, Kazuki; Komatsu, Atsushi; Kitatsuji, Yoshihiro; Watanabe, Masayuki
European Journal of Inorganic Chemistry, 27(14), p.e202300787_1 - e202300787_7, 2024/05
Times Cited Count:1 Percentile:0.00(Chemistry, Inorganic & Nuclear)Electrochemical behavior of U O
O
 /U
/U O
O
 in 1-ethyl-3-methylimidazolium bis(trifluoromethyl)sulfonylamide ([emim]Tf
in 1-ethyl-3-methylimidazolium bis(trifluoromethyl)sulfonylamide ([emim]Tf N) ionic liquid was studied to clarify what are required to attain its redox reversibility for utilizing depleted U as an electrode active material in a redox flow battery. As a result, reversibility of the U
N) ionic liquid was studied to clarify what are required to attain its redox reversibility for utilizing depleted U as an electrode active material in a redox flow battery. As a result, reversibility of the U O
O
 /U
/U O
O
 redox reaction was successfully achieved in use of a glassy carbon working electrode under presence of Cl
redox reaction was successfully achieved in use of a glassy carbon working electrode under presence of Cl in [emim]Tf
in [emim]Tf N. To improve diffusivity of solutes, [emim]Tf
N. To improve diffusivity of solutes, [emim]Tf N diluted with an auxiliary molecular solvent, N,N-dimethylformamide (DMF). We have succeeded in demonstrating a reversible redox reaction of [U
N diluted with an auxiliary molecular solvent, N,N-dimethylformamide (DMF). We have succeeded in demonstrating a reversible redox reaction of [U O
O Cl
Cl ]
] + e
+ e = [U
= [U O
O Cl
Cl ]
] in the 50:50 v/v [emim]Tf
in the 50:50 v/v [emim]Tf N-DMF liquid electrolyte containing Cl
N-DMF liquid electrolyte containing Cl .
.
 Sr in highly radioactive aqueous samples via conversion to a kinetically stable 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid complex followed by concentration-separation-fractionation based on capillary electrophoresis-liquid scintillation
Sr in highly radioactive aqueous samples via conversion to a kinetically stable 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid complex followed by concentration-separation-fractionation based on capillary electrophoresis-liquid scintillationOuchi, Kazuki; Haraga, Tomoko; Hirose, Kazuki*; Kurosawa, Yuika*; Sato, Yoshiyuki; Shibukawa, Masami*; Saito, Shingo*
Analytica Chimica Acta, 1298, p.342399_1 - 342399_7, 2024/04
Times Cited Count:1 Percentile:40.01(Chemistry, Analytical)Given that conventional methods of high-dose sample analysis pose substantial exposure risks and generate large amounts of secondary radioactive waste, faster procedures allowing for decreased radiation emission are highly desirable. To address this need, we developed a  Sr
Sr quantitation technique that is based on liquid scintillation counting-coupled capillary transient isotachophoresis (ctITP) with two-point detection and relies on the rapid concentration, separation, and fractionation of 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid (DOTA)-complexed
quantitation technique that is based on liquid scintillation counting-coupled capillary transient isotachophoresis (ctITP) with two-point detection and relies on the rapid concentration, separation, and fractionation of 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid (DOTA)-complexed  Sr
Sr in a single run. This method, which allows for the handling of high-dose radioactive specimens at the microliter level and is substantially faster than conventional ion-exchange protocols, was used to selectively quantify
in a single run. This method, which allows for the handling of high-dose radioactive specimens at the microliter level and is substantially faster than conventional ion-exchange protocols, was used to selectively quantify  Sr
Sr in real high-dose waste. The successful concentration-separation in ctITP was ascribed to the inertness of the Sr-DOTA complex to dissociation.
in real high-dose waste. The successful concentration-separation in ctITP was ascribed to the inertness of the Sr-DOTA complex to dissociation.
 Ac and its uncertainty through the
Ac and its uncertainty through the  Ra(n,2n) reaction in the experimental fast reactor Joyo
Ra(n,2n) reaction in the experimental fast reactor JoyoSasaki, Yuto*; Sano, Aaru; Sasaki, Shinji; Iwamoto, Nobuyuki; Ouchi, Kazuki; Kitatsuji, Yoshihiro; Takaki, Naoyuki*; Maeda, Shigetaka
Journal of Nuclear Science and Technology, 61(4), p.509 - 520, 2024/04
Times Cited Count:5 Percentile:73.39(Nuclear Science & Technology) Ac is attracting attention as an alpha-emitting medical radioisotope. Since its demand is expected to increase, domestic production of
Ac is attracting attention as an alpha-emitting medical radioisotope. Since its demand is expected to increase, domestic production of  Ac is required from the viewpoint of Japan's medical research and economic security. To establish the technical bases for the
Ac is required from the viewpoint of Japan's medical research and economic security. To establish the technical bases for the  Ac production, JAEA has evaluated the radioactivity that can be produced in the experimental fast reactor Joyo and designed the concept that upgrades the existing facilities for transporting the irradiated target from Joyo to a neighboring PIE facility rapidly. Efficient
Ac production, JAEA has evaluated the radioactivity that can be produced in the experimental fast reactor Joyo and designed the concept that upgrades the existing facilities for transporting the irradiated target from Joyo to a neighboring PIE facility rapidly. Efficient  Ac Separation from
Ac Separation from  Ra irradiated in a fast reactor was studied. Ba and La were used as alternatives to Ra and Ac, respectively. By using DGA resin as an adsorbent, it can be expected that Ra and impurities generated by irradiation will be removed and Ac will be isolated. This study has revealed that Joyo can sufficiently produce
Ra irradiated in a fast reactor was studied. Ba and La were used as alternatives to Ra and Ac, respectively. By using DGA resin as an adsorbent, it can be expected that Ra and impurities generated by irradiation will be removed and Ac will be isolated. This study has revealed that Joyo can sufficiently produce  Ac as a raw material for pharmaceuticals.
Ac as a raw material for pharmaceuticals.
Ouchi, Kazuki
Hosha Kagaku, (49), p.3 - 7, 2024/03
I introduce the elucidation of the deposition following the oxidation state of uranium and the electrochemical behavior of uranium(IV) chloride in an ionic liquid-organic mixture, as a basic study of in-solution reactions. In addition, I introduce the development of separation methods for actinides using a microchemical chip and polyacrylamide gel electrophoresis, as an applied study for quantitative analytical methods for small amounts of samples.
Morii, Shiori; Yomogida, Takumi; Asai, Shiho*; Ouchi, Kazuki; Oka, Toshitaka; Kitatsuji, Yoshihiro
KEK Proceedings 2023-2, p.132 - 137, 2023/11
New analytical method of a combination of laser ablation inductively coupled plasma mass spectrometry (LA-ICP-MS) and isotope dilution mass spectrometry (IDMS) for quantification of Zr isotopes in a solid sample was investigated. Solid Zr-isotope reference was added to a simulated radioactive waste sample as a spike, then Zr isotope ratio was measured by LA-ICP-MS. As a result, we successfully quantify Zr isotopes in the simulated radioactive waste sample by new IDMS. There is a possibility that this new method can be applied for quantification of Zr-93 in difficult to dissolve radioactive wastes.
Morii, Shiori; Yomogida, Takumi; Asai, Shiho*; Ouchi, Kazuki; Oka, Toshitaka; Kitatsuji, Yoshihiro
Bunseki Kagaku, 72(10.11), p.441 - 448, 2023/10
Rapid analytical method for the determination of Zr-93 in radioactive wastes has been developed. Laser ablation (LA)-ICP-MS was applied to the analysis of Zr isotopes in simulated high-level radioactive waste (HLW). Sample preparation time was dramatically reduced by using a DGA resin as the adsorbent for Zr. Direct quantification of Zr isotopes in this resin sample was carried out by LA-ICP-MS. Laser settings were optimized to obtain a reliable isotope ratio of the sample by LA-ICP-MS. Quantification of Zr isotopes in the simulated HLW solution by isotope dilution mass spectrometry (IDMS) was examined. The amount of Zr-90 in the sample obtained by IDMS corresponded to a value calculated from the given concentration of Zr in the sample within uncertainty. Thus, this method can be applied for the quantification of Zr-93 in radioactive wastes.
Ouchi, Kazuki; Matsumura, Daiju; Tsuji, Takuya; Kobayashi, Toru; Otobe, Haruyoshi; Kitatsuji, Yoshihiro
RSC Advances (Internet), 13(24), p.16321 - 16326, 2023/05
Times Cited Count:1 Percentile:11.04(Chemistry, Multidisciplinary)We clarified the chemical state transformation of deposits following the reduction of uranyl ion (U O
O
 ) from the results of electrochemical quartz crystal microbalance, impedance spectra and X-ray absorption fine structure measurements. We propose the following deposition mechanism: (1) U
) from the results of electrochemical quartz crystal microbalance, impedance spectra and X-ray absorption fine structure measurements. We propose the following deposition mechanism: (1) U is formed by the disproportionation of U
is formed by the disproportionation of U . (2) U
. (2) U forms U
forms U hydroxide deposits, and (3) finally, the hydroxide deposits transform into U
hydroxide deposits, and (3) finally, the hydroxide deposits transform into U oxide, generally having a larger electrical resistance than the former.
oxide, generally having a larger electrical resistance than the former.
Iwase, Hiroki*; Akamatsu, Masaaki*; Inamura, Yasuhiro; Sakaguchi, Yoshifumi*; Morikawa, Toshiaki*; Kasai, Satoshi*; Ouchi, Keiichi*; Kobayashi, Kazuki*; Sakai, Hideki*
Journal of Applied Crystallography, 56(1), p.110 - 115, 2023/02
Times Cited Count:5 Percentile:77.05(Chemistry, Multidisciplinary)With the increasing importance of light-responsive materials, it is vital to analyze the relationship between function and structural changes induced by light irradiation. Small-angle scattering (SAS) is effective for such structural analysis. However, quantitatively capturing local molecular structure formation and molecular reactions at a scale of less than 1 nm via SAS is difficult. In this study, to analyze the structure of non-equilibrium phenomena in light-responsive materials, a new sample environment has been developed for a time-of-flight small- and wide-angle neutron scattering instrument (TAIKAN), comprising a UV-Vis irradiation system, UV-Vis absorption measurement equipment and photodetector. Simultaneous measurement of small-angle neutron scattering and UV-Vis absorption was achieved. This system was used to demonstrate the in situ observation of UV-Vis irradiation-induced structural change of micelles formed by a light-responsive surfactant sample in an aqueous solution.
Yamagata, Kazuhito*; Ouchi, Kazuki; Marumo, Kazuki*; Tasaki-Handa, Yuiko*; Haraga, Tomoko; Saito, Shingo*
Inorganic Chemistry, 62(2), p.730 - 738, 2023/01
Times Cited Count:3 Percentile:42.77(Chemistry, Inorganic & Nuclear)The inert NpO
 complex with a fluorescein-modified phenanthroline-2,9-dicarboxylic acid was found by kinetic selection using polyacrylamide gel electrophoresis (PAGE) from a small chemical library. The small spontaneous dissociation rate constant of 8
complex with a fluorescein-modified phenanthroline-2,9-dicarboxylic acid was found by kinetic selection using polyacrylamide gel electrophoresis (PAGE) from a small chemical library. The small spontaneous dissociation rate constant of 8 10
10 s
s (the half-life of 23 hours) was determined. This is the singly-charged NpO
(the half-life of 23 hours) was determined. This is the singly-charged NpO
 complex exhibiting unusual kinetic inertness in aqueous solution, one million times slower than widely accepted fast kinetics of neptunyl complexes. Selective fluorescence detection of NpO
complex exhibiting unusual kinetic inertness in aqueous solution, one million times slower than widely accepted fast kinetics of neptunyl complexes. Selective fluorescence detection of NpO
 was achieved in PAGE with a detection limit of 68 pmol dm
was achieved in PAGE with a detection limit of 68 pmol dm (17 fg). This system was successfully applied to simulated spent nuclear fuel and high-level radioactive waste samples.
(17 fg). This system was successfully applied to simulated spent nuclear fuel and high-level radioactive waste samples.
 -edge to assess the U(V) electronic structure in FeUO
-edge to assess the U(V) electronic structure in FeUO
Yomogida, Takumi; Akiyama, Daisuke*; Ouchi, Kazuki; Kumagai, Yuta; Higashi, Kotaro*; Kitatsuji, Yoshihiro; Kirishima, Akira*; Kawamura, Naomi*; Takahashi, Yoshio*
Inorganic Chemistry, 61(50), p.20206 - 20210, 2022/12
Times Cited Count:7 Percentile:59.79(Chemistry, Inorganic & Nuclear)FeUO was studied to clarify the electronic structure of U(V) in a metal monouranate compound. We obtained the peak splitting of HERFD-XANES spectra utilizing high-energy-resolution fluorescence detection-X-ray absorption near edge structure (HERFD-XANES) spectroscopy at the U L
was studied to clarify the electronic structure of U(V) in a metal monouranate compound. We obtained the peak splitting of HERFD-XANES spectra utilizing high-energy-resolution fluorescence detection-X-ray absorption near edge structure (HERFD-XANES) spectroscopy at the U L -edge, which is a novel technique in the U(V) compounds. Theoretical calculations revealed that the peak splitting was caused by splitting the 6d orbital of U(V). Such distinctive electronic states are of major interest to researchers and engineers working in various fields, from fundamental physics to the nuclear industry and environmental sciences for actinide elements.
-edge, which is a novel technique in the U(V) compounds. Theoretical calculations revealed that the peak splitting was caused by splitting the 6d orbital of U(V). Such distinctive electronic states are of major interest to researchers and engineers working in various fields, from fundamental physics to the nuclear industry and environmental sciences for actinide elements.
 Fe
Fe O
O solid solutions under high-pressure and high-temperature conditions
solid solutions under high-pressure and high-temperature conditionsYamanaka, Takamitsu*; Hirao, Naohisa*; Nakamoto, Yuki*; Mikouchi, Takashi*; Hattori, Takanori; Komatsu, Kazuki*; Mao, H.-K.*
Physics and Chemistry of Minerals, 49(10), p.41_1 - 41_14, 2022/10
Times Cited Count:1 Percentile:7.69(Materials Science, Multidisciplinary)Magnetic and crystal structure of Mn Fe
Fe O
O solid solutions under high-PT conditions are investigated by neutron diffraction and synchrotron M
solid solutions under high-PT conditions are investigated by neutron diffraction and synchrotron M ssbauer spectroscopy. The ferrimagnetic-paramagnetic transition and tetragonal-cubic transition of Mn
ssbauer spectroscopy. The ferrimagnetic-paramagnetic transition and tetragonal-cubic transition of Mn FeO
FeO spinel occur at 100
spinel occur at 100 C and 180
C and 180 C, respectively, suggesting both the transitions are not coupled. The structure transition temperature decreases with pressure. M
C, respectively, suggesting both the transitions are not coupled. The structure transition temperature decreases with pressure. M ssbauer experiments and neutron diffraction revealed that the Fe
ssbauer experiments and neutron diffraction revealed that the Fe occupancy in tetrahedral site increases increase with pressure, suggesting Mn
occupancy in tetrahedral site increases increase with pressure, suggesting Mn FeO
FeO phase approaches inverse spinel. Magnetic structure refinement clarified paramagnetic and ferrimagnetic structure of MnFe
phase approaches inverse spinel. Magnetic structure refinement clarified paramagnetic and ferrimagnetic structure of MnFe O
O and Mn
and Mn FeO
FeO . These spinels transform into high-pressure orthorhombic phases at 18.4 and 14.0 GPa, respectively, indicating lower transition pressure with increasing Mn content.
. These spinels transform into high-pressure orthorhombic phases at 18.4 and 14.0 GPa, respectively, indicating lower transition pressure with increasing Mn content.
Yomogida, Takumi; Ouchi, Kazuki; Oka, Toshitaka; Kitatsuji, Yoshihiro; Koma, Yoshikazu; Konno, Katsuhiro*
Scientific Reports (Internet), 12(1), p.7191_1 - 7191_10, 2022/05
Times Cited Count:9 Percentile:56.94(Multidisciplinary Sciences)Particles containing alpha ( ) nuclides were identified from sediment in stagnant water at the torus room of the Fukushima Dai-ichi Nuclear Power Station (FDiNPS)'s Unit 2 reactor. Several uranium-bearing particles were identified by SEM observation. These particles contained Zr and other elements which constituted fuel cladding and structural materials. The
) nuclides were identified from sediment in stagnant water at the torus room of the Fukushima Dai-ichi Nuclear Power Station (FDiNPS)'s Unit 2 reactor. Several uranium-bearing particles were identified by SEM observation. These particles contained Zr and other elements which constituted fuel cladding and structural materials. The  U/
U/ U isotope ratio in the solid fractions that included U particles was consistent with the nuclear fuel in the Unit 2 reactor, which indicated that the U particles had been derived from nuclear fuel. The particles with alpha-emitters detected by alpha track analysis were several tens to several hundred
U isotope ratio in the solid fractions that included U particles was consistent with the nuclear fuel in the Unit 2 reactor, which indicated that the U particles had been derived from nuclear fuel. The particles with alpha-emitters detected by alpha track analysis were several tens to several hundred  m in size. The EDX spectra showed that these particles mainly comprised iron, which indicated Pu, Am, and Cm were adsorbed on the Fe-baring particles. This study clarifies that the major morphologies of U and other
m in size. The EDX spectra showed that these particles mainly comprised iron, which indicated Pu, Am, and Cm were adsorbed on the Fe-baring particles. This study clarifies that the major morphologies of U and other  -nuclides were differed in the sediment of stagnant water in the torus room of FDiNPS's Unit 2 reactor.
-nuclides were differed in the sediment of stagnant water in the torus room of FDiNPS's Unit 2 reactor.
Ouchi, Kazuki; Tsukahara, Takehiko*; Brandt, A.*; Muto, Yuki*; Nabatame, Nozomi*; Kitatsuji, Yoshihiro
Analytical Sciences, 37(12), p.1789 - 1794, 2021/12
Times Cited Count:1 Percentile:3.83(Chemistry, Analytical)We attempted to scale down a separation process of uranium (U) using the microchip column loaded with anion exchange resin to develop safety and waste-reducing separation technique. The ideal separation performance of U was obtained by the properly design of a microchannel. The concentration of U in seawater as a real-world sample could be quantified with the prepared microchip column. It indicates that the microchip column is sufficiently practical. Compared to separation of U with a general column, the column size was successfully scaled down to  1/5000.
1/5000.
Ouchi, Kazuki; Komatsu, Atsushi; Takao, Koichiro*; Kitatsuji, Yoshihiro; Watanabe, Masayuki
Chemistry Letters, 50(6), p.1169 - 1172, 2021/06
Times Cited Count:2 Percentile:4.05(Chemistry, Multidisciplinary)The electrochemical behavior of uranium (IV) tetrachloride in ionic liquid-DMF mixture was studied for first time in order to build a redox flow battery (RFB) using U as an electrode active material. We found a quasi-reversible U /U
/U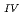 couple that could be applied to the anode reaction of the RFB.
couple that could be applied to the anode reaction of the RFB.
Haraga, Tomoko; Ouchi, Kazuki; Sato, Yoshiyuki; Hoshino, Hitoshi*; Tanana, Rei*; Fujihara, Takashi*; Kurokawa, Hideki*; Shibukawa, Masami*; Ishimori, Kenichiro; Kameo, Yutaka; et al.
Analytica Chimica Acta, 1032, p.188 - 196, 2018/11
Times Cited Count:14 Percentile:46.22(Chemistry, Analytical)The development of safe, rapid and highly sensitive analytical methods for radioactive samples, especially actinide (An) ions, represents an important challenge. Here we propose a methodology for selecting appropriate emissive probes for An ions with very low consumption and emission of radioactivity by capillary electrophoresis-laser-induced fluorescence detection (CE-LIF), using a small chemical library of probes with eight different chelating moieties. It was found that the emissive probe, which possesses the tetradentate chelating moiety, was suitable for detecting uranyl ions. The detection limit for the uranyl-probe complex using CE-LIF combined with dynamic ternary complexation and on-capillary concentration techniques was determined to be 0.7 ppt. This method was successfully applied to real radioactive liquid samples collected from nuclear facilities.
Ouchi, Kazuki; Otobe, Haruyoshi; Kitatsuji, Yoshihiro; Yamamoto, Masahiro
ECS Transactions, 75(27), p.51 - 57, 2017/01
Times Cited Count:1 Percentile:36.09(Electrochemistry)We investigated the deposition of U(IV) following a valence change of U as electrodeposition using an electrochemical quartz crystal microbalance (EQCM). When measurements of the reduction of U(VI) in a weak acid solution were performed, deposits of U(IV) were observed on the electrode surface. From deposition rates, pH dependence of them, and oxidation potentials of deposits, we proposed the following deposition mechanism. The deposition is divided into the three phases; First, in the induction phase, U(IV) produced by the disproportionation forms U(IV) hydroxide nucleus. Next, in the growth phase, U(IV) deposits begin to grow. In this phase, the deposits catalyze the reduction of U(V) to U(IV), resulting an increase of the reduction current. Finally, in the transformation phase, U(IV) hydroxide species transform into U dioxide having more stable state.
Sato, Takahiro*; Iwasaki, Atsushi*; Ishibashi, Kazuki*; Okino, Tomoya*; Yamanouchi, Kaoru*; Adachi, Junichi*; Yagishita, Akira*; Yazawa, Hiroki*; Kannari, Fumihiko*; Aoyama, Makoto; et al.
Europhysics News, 42(5), P. 10, 2011/09
The resonant and non-resonant two-photon single ionization processes of He were investigated using intense free electron laser light in the extreme ultraviolet (XUV) region (53.4-61.4 nm) covering the 1s-2p and 1s-3p resonant transitions of He. On the basis of the dependences of the yield of He on the XUV light-field intensity at 53.4, 58.4, 56.0 and 61.4 nm, the absolute values of the two-photon ionization cross sections of He at the four different wavelengths and their dependence on the light-field intensity were determined for the first time.
on the XUV light-field intensity at 53.4, 58.4, 56.0 and 61.4 nm, the absolute values of the two-photon ionization cross sections of He at the four different wavelengths and their dependence on the light-field intensity were determined for the first time.
Sato, Takahiro*; Iwasaki, Atsushi*; Ishibashi, Kazuki*; Okino, Tomoya*; Yamanouchi, Kaoru*; Adachi, Junichi*; Yagishita, Akira*; Yazawa, Hiroki*; Kannari, Fumihiko*; Aoyama, Makoto; et al.
Journal of Physics B; Atomic, Molecular and Optical Physics, 44(16), p.161001_1 - 161001_5, 2011/08
Times Cited Count:39 Percentile:83.86(Optics)The resonant and non-resonant two-photon single ionization processes of He were investigated using intense free electron laser light in the extreme ultraviolet (XUV) region (53.4-61.4 nm) covering the 1s-2p and 1s-3p resonant transitions of He. On the basis of the dependences of the yield of He on the XUV light-field intensity at 53.4, 58.4, 56.0 and 61.4 nm, the absolute values of the two-photon ionization cross sections of He at the four different wavelengths and their dependence on the light-field intensity were determined for the first time.
on the XUV light-field intensity at 53.4, 58.4, 56.0 and 61.4 nm, the absolute values of the two-photon ionization cross sections of He at the four different wavelengths and their dependence on the light-field intensity were determined for the first time.