Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
 acidic solutions
acidic solutionsYokoyama, Akihiko*; Kitayama, Yuta*; Fukuda, Yoshiki*; Kikunaga, Hidetoshi*; Murakami, Masashi*; Komori, Yukiko*; Yano, Shinya*; Haba, Hiromitsu*; Tsukada, Kazuaki; Toyoshima, Atsushi*
Radiochimica Acta, 107(1), p.27 - 32, 2019/01
Times Cited Count:1 Percentile:10.81(Chemistry, Inorganic & Nuclear)Oe, Kazuhiro*; Attallah, M. F.*; Asai, Masato; Goto, Naoya*; Gupta, N. S.*; Haba, Hiromitsu*; Huang, M.*; Kanaya, Jumpei*; Kaneya, Yusuke*; Kasamatsu, Yoshitaka*; et al.
Journal of Radioanalytical and Nuclear Chemistry, 303(2), p.1317 - 1320, 2015/02
Times Cited Count:10 Percentile:64.26(Chemistry, Analytical)A new technique for continuous dissolution of nuclear reaction products transported by a gas-jet system was developed for superheavy element (SHE) chemistry. In this technique, a hydrophobic membrane is utilized to separate an aqueous phase from the gas phase. With this technique, the dissolution efficiencies of short-lived radionuclides of 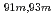 Mo and
Mo and 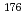 W were measured. Yields of more than 80% were observed for short-lived radionuclides at aqueous-phase flow rates of 0.1-0.4 mL/s. The gas flow-rate had no influence on the dissolution efficiency within the studied flow range of 1.0-2.0 L/min. These results show that this technique is applicable for on-line chemical studies of SHEs in the liquid phase.
W were measured. Yields of more than 80% were observed for short-lived radionuclides at aqueous-phase flow rates of 0.1-0.4 mL/s. The gas flow-rate had no influence on the dissolution efficiency within the studied flow range of 1.0-2.0 L/min. These results show that this technique is applicable for on-line chemical studies of SHEs in the liquid phase.
 SO
SO /HNO
/HNO mixed solution
mixed solutionLi, Z.*; Toyoshima, Atsushi; Asai, Masato; Tsukada, Kazuaki; Sato, Tetsuya; Sato, Nozomi; Kikuchi, Takahiro; Nagame, Yuichiro; Sch del, M.; Pershina, V.*; et al.
del, M.; Pershina, V.*; et al.
Radiochimica Acta, 100(3), p.157 - 164, 2012/03
Times Cited Count:14 Percentile:68.93(Chemistry, Inorganic & Nuclear)Matsushita, Yoshitaka*; Izumi, Fujio*; Kobayashi, Kiyoshi*; Igawa, Naoki; Kitazawa, Hideaki*; Oyama, Yukiko*; Miyoshi, Shogo*; Yamaguchi, Shu*
Nuclear Instruments and Methods in Physics Research A, 600(1), p.319 - 321, 2009/02
Times Cited Count:22 Percentile:80.29(Instruments & Instrumentation)The neutron powder diffraction data of the apatite-type La-silicate La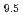 Si
Si O
O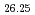 was measured at 10 K and the Rietveld refinement on the basis of hexagonal P6
was measured at 10 K and the Rietveld refinement on the basis of hexagonal P6 /m was successfully carried out. The obtained cell parameters were
/m was successfully carried out. The obtained cell parameters were  =0.971297(7) nm, and
=0.971297(7) nm, and  = 0.717950(6) nm. On the Rietveld refinement supported by maximum entropy method, the interstitial oxygen positions show randomly distribution around La2 site, and they may give the driving-force of high ionic conductivity to the main ionic conduction site O4.
= 0.717950(6) nm. On the Rietveld refinement supported by maximum entropy method, the interstitial oxygen positions show randomly distribution around La2 site, and they may give the driving-force of high ionic conductivity to the main ionic conduction site O4.
Kobayashi, Kiyoshi*; Matsushita, Yoshitaka*; Igawa, Naoki; Izumi, Fujio*; Nishimura, Chikashi*; Miyoshi, Shogo*; Oyama, Yukiko*; Yamaguchi, Shu*
Solid State Ionics, 179(38), p.2209 - 2215, 2008/11
Times Cited Count:24 Percentile:67.72(Chemistry, Physical)A novel synthesis method of lanthanum silicate apatite was developed by a sol-gel method using an aqueous solution system. The processes of apatite phase formation were investigated by XRD and TG-DTA. Lanthanum dioxide carbonate was found in the sample below 873 K and lanthanum silicate apatite was formed above 1073 K. It was founded that the profiles of powder XRD and neutron diffraction could be refined by the oxy-apatite structure with the space group  6
6 /
/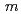 .
.
Kobayashi, Kiyoshi*; Yoshida, Akira*; Matsushita, Yoshitaka*; Nishimura, Chikashi*; Oyama, Yukiko*; Miyoshi, Shogo*; Izumi, Fujio*; Igawa, Naoki; Yamaguchi, Shu*
no journal, ,
Conduction properties of oxide ions and crystal structure refinement of La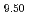 (SiO
(SiO )
) O
O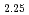 have been made by A.C.-impedance, electro-motive force of oxygen concentration cell, X-ray and neutron diffraction methods using the sample fabricated by the sol-gel method. The crystal structure of the sample can be refined by structural model with
have been made by A.C.-impedance, electro-motive force of oxygen concentration cell, X-ray and neutron diffraction methods using the sample fabricated by the sol-gel method. The crystal structure of the sample can be refined by structural model with  6
6 /
/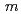 space group. Oxide ion conductivity is found to be predominant in wide oxygen partial pressure and temperature range based on the conductivity and electro-motive force measurements.
space group. Oxide ion conductivity is found to be predominant in wide oxygen partial pressure and temperature range based on the conductivity and electro-motive force measurements.
 SO
SO /HNO
/HNO mixed solution ([H
mixed solution ([H ] = 1.0 M)
] = 1.0 M)Li, Z.; Toyoshima, Atsushi; Asai, Masato; Tsukada, Kazuaki; Sato, Tetsuya; Sato, Nozomi; Kikuchi, Takahiro; Sch del, M.*; Nagame, Yuichiro; Liang, X. H.*; et al.
del, M.*; Nagame, Yuichiro; Liang, X. H.*; et al.
no journal, ,
Cation-exchange behavior of  Rf (T
Rf (T = 78 s) produced in the
= 78 s) produced in the  Cm(
Cm( O, 5n) reaction was studied on a "one-atom-at-a-time" scale in H
O, 5n) reaction was studied on a "one-atom-at-a-time" scale in H SO
SO (0.15-0.69 M)/HNO
(0.15-0.69 M)/HNO mixed solutions ([H
mixed solutions ([H ] = 1.0 M) using an automated ion-exchange separation apparatus coupled with the detection system for alpha-spectroscopy. It was found that adsorption probability (%ads) of
] = 1.0 M) using an automated ion-exchange separation apparatus coupled with the detection system for alpha-spectroscopy. It was found that adsorption probability (%ads) of  Rf on cation-exchange resin decreases with increasing [HSO
Rf on cation-exchange resin decreases with increasing [HSO
 ], showing a successive formation of its sulfate complexes. Rf exhibited much weaker formation of the complexes than the lighter homologues Zr and Hf, which is qualitatively in good agreement with theoretical calculations including relativistic effects.
], showing a successive formation of its sulfate complexes. Rf exhibited much weaker formation of the complexes than the lighter homologues Zr and Hf, which is qualitatively in good agreement with theoretical calculations including relativistic effects.
 SO
SO /HNO
/HNO mixed solution ([H
mixed solution ([H ] = 1.0 M)
] = 1.0 M)Li, Z.; Toyoshima, Atsushi; Asai, Masato; Tsukada, Kazuaki; Sato, Tetsuya; Sato, Nozomi; Sch del, M.*; Nagame, Yuichiro; Liang, X. H.*; Kasamatsu, Yoshitaka*; et al.
del, M.*; Nagame, Yuichiro; Liang, X. H.*; Kasamatsu, Yoshitaka*; et al.
no journal, ,
Cation-exchange behavior of  Rf (T
Rf (T = 78 s) produced in the
= 78 s) produced in the  Cm(
Cm( O,5n) reaction was studied on a "one-atom-at-a-time" scale in 0.15-0.69 M H
O,5n) reaction was studied on a "one-atom-at-a-time" scale in 0.15-0.69 M H SO
SO /HNO
/HNO mixed solutions ([H
mixed solutions ([H ] = 1.0 M) using an automated ion-exchange separation apparatus coupled with a detection system for alpha-spectroscopy (AIDA). It was found that adsorption probabilities (%ads) of Rf on the cation-exchange resin decrease with increasing [HSO
] = 1.0 M) using an automated ion-exchange separation apparatus coupled with a detection system for alpha-spectroscopy (AIDA). It was found that adsorption probabilities (%ads) of Rf on the cation-exchange resin decrease with increasing [HSO
 ], showing successive formation of Rf sulfate complexes. Rf exhibits a weaker complex formation than the lighter homologues Zr and Hf.
], showing successive formation of Rf sulfate complexes. Rf exhibits a weaker complex formation than the lighter homologues Zr and Hf.
Toyoshima, Atsushi; Miyashita, Sunao*; Asai, Masato; Sato, Tetsuya; Kaneya, Yusuke; Tsukada, Kazuaki; Kitatsuji, Yoshihiro; Nagame, Yuichiro; Sch del, M.; Lerum, H. V.*; et al.
del, M.; Lerum, H. V.*; et al.
no journal, ,
To carry out a continuous reduction experiment of Sg with the low production rates and the short half-life, we are developing a new chemistry assembly consisting of a membrane degasser (MDG), a flow electrolytic column (FEC), the continuous liquid-liquid extraction apparatus, and the liquid scintillation counting system (SISAK). Recently, we have begun preparatory studies with Mo and W isotopes. Aqueous solution dissolving Mo and W was successfully separated from a carrier gas. Redox couples of Mo(VI)/Mo(V) and W(VI)/W(V) in HCl have been characterized for their macro amounts. Extraction behavior of Mo(VI) and W(VI) between toluene containing hinokitiol (HT) and HCl was successfully observed by a batch method. On-line extractions of short-lived Mo and W were also carried out using SISAK and MDG. In the symposium, our present status of the preparation with Mo and W will be presented.
Toyoshima, Atsushi; Asai, Masato; Attallah, M. F.*; Goto, Naoya*; Gupta, N. S.*; Haba, Hiromitsu*; Huang, M.*; Kanaya, Jumpei*; Kaneya, Yusuke; Kasamatsu, Yoshitaka*; et al.
no journal, ,
Towards electrolytic reduction of Sg, batch-wise electrolytic reduction of carrier-free 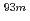 Mo and
Mo and 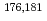 W radiotracers was studied using a flow electrolytic column (FEC). The electrolyzed samples from a FEC were chemically analyzed by solvent extraction with TOA and HDEHP to separate and identify reduced species from the stable Mo(VI) and W(VI) ones based on their different extraction behavior.
W radiotracers was studied using a flow electrolytic column (FEC). The electrolyzed samples from a FEC were chemically analyzed by solvent extraction with TOA and HDEHP to separate and identify reduced species from the stable Mo(VI) and W(VI) ones based on their different extraction behavior. 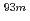 Mo and
Mo and 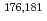 W were applied as radiotracers. We also performed cyclic voltammetry and UV/Vis absorption spectrometry of macro amounts of Mo and W in acidic solutions to obtain information on redox reactions of these elements under given conditions. In the conference, the present status of the preparatory reduction experiments with Mo and W will be presented.
W were applied as radiotracers. We also performed cyclic voltammetry and UV/Vis absorption spectrometry of macro amounts of Mo and W in acidic solutions to obtain information on redox reactions of these elements under given conditions. In the conference, the present status of the preparatory reduction experiments with Mo and W will be presented.
Toyoshima, Atsushi; Miyashita, Sunao*; Oe, Kazuhiro*; Kitayama, Yuta*; Lerum, H. V.*; Goto, Naoya*; Kaneya, Yusuke; Komori, Yukiko*; Mitsukai, Akina*; Vascon, A.; et al.
no journal, ,
no abstracts in English
Toyoshima, Atsushi; Oe, Kazuhiro*; Asai, Masato; Attallah, M. F.*; Goto, Naoya*; Gupta, N. S.*; Haba, Hiromitsu*; Kaneko, Masashi*; Kaneya, Yusuke; Kasamatsu, Yoshitaka*; et al.
no journal, ,
Due to short half-lives less than 10 s and extremely low production rates, transactinide elements heavier than seaborgium (Sg) are produced on an atom per hour scale. Therefore, a continuous rapid chemistry assembly is required to study aqueous-phase chemistry of these heaviest elements. In the present study, we started developments of a continuous chemistry assembly. Our first attempt was made in on-line experiments with Mo and W, lighter homologs of Sg, to optimize a chemistry assembly consisting of a newly developed membrane degasser as an interface between gas-jet and aqueous phase, a flow electrolytic column apparatus utilized to control oxidation states of Mo and W ions, and the continuous liquid-liquid extraction apparatus of SISAK for separation. In the conference, present status of the developments will be presented.
 solution for identification of fluoride species of Db
solution for identification of fluoride species of DbToyoshima, Atsushi; Mitsukai, Akina; Murakami, Masashi*; Sato, Daisuke*; Motoyama, Risa*; Oe, Kazuhiro*; Komori, Yukiko*; Haba, Hiromitsu*; Asai, Masato; Tsukada, Kazuaki; et al.
no journal, ,
The purpose of the present study is characterization of fluoride complexes of Db. In this study, we investigated anion-exchange behavior of Nb and Ta, lighter homologs of Db, in 1.0-24 M HF/2.0 M HNO solutions as a model experiment of Db.
solutions as a model experiment of Db. 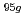 Nb and
Nb and 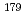 Ta produced at JAEA tandem accelerator and RIKEN AVF cyclotron, respectively, were prepared as non-carrier-added radiotracers by an ion exchange method. Batch experiments were performed with anion-exchange resin. As a result, distribution coefficients (
Ta produced at JAEA tandem accelerator and RIKEN AVF cyclotron, respectively, were prepared as non-carrier-added radiotracers by an ion exchange method. Batch experiments were performed with anion-exchange resin. As a result, distribution coefficients (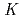
 ) of Nb showed a sharp increase at
) of Nb showed a sharp increase at  6 M HF while those of Ta had a monotonic decrease as increasing HF concentration. This suggests that Nb forms fluoride complexes from oxy fluoride one at
6 M HF while those of Ta had a monotonic decrease as increasing HF concentration. This suggests that Nb forms fluoride complexes from oxy fluoride one at  6 M HF and Ta exists as fluoride complexes under the conditions. It is expected that chemical species of Db fluorides are characterized in its anion-exchange experiments under the present conditions.
6 M HF and Ta exists as fluoride complexes under the conditions. It is expected that chemical species of Db fluorides are characterized in its anion-exchange experiments under the present conditions.
Saeki, Morihisa*; Oba, Hironori*; Taguchi, Tomitsugu*; Yokoyama, Jun*; Asai, Shiho; Yomogida, Takumi; Hanzawa, Yukiko; Nakashima, Nobuaki*
no journal, ,
We are developing separation of platinum-group metals from a solution of high-level radioactive waste by laser-induced particle formation. In this report, we will show method and proof-of-principle experiments of the separation using the laser-induced particle formation.