Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Nguyen, T. H.*; Le Ba, T.*; Tran, C. T.*; Nguyen, T. T.*; Doan, T. T. T.*; Do, V. K.; Watanabe, Masayuki; Pham, Q. M.*; Hoang, S. T.*; Nguyen, D. V.*; et al.
Hydrometallurgy, 213, p.105933_1 - 105933_11, 2022/08
Times Cited Count:9 Percentile:81.6(Metallurgy & Metallurgical Engineering)A continuous counter-current extraction for the selective recovery of thorium (Th) and uranium (U) from the Yen Phu (Vietnam) rare earth concentrate leach solutions was systematically studied. The primary amine N1923 was used as an extractant which was prepared in the isoparaffin IP-2028 diluent. Thorium and uranium were selectively recovered in a hydrometallurgical circuit established by continuous mixer-settler extraction, scrubbing, and back-extraction at the laboratory scale. The desired purity of Th and U can be achieved by managing the volume ratio of organic to aqueous phase (O/A ratio) in the corresponding steps. Highly pure Th and U were recovered from the pregnant back-extraction liquor and the raffinate, respectively, which have satisfactory properties for further processing of the subsequent nuclear materials.
 Ln
Ln O
O (Ln = Gd, Er) solid solution
(Ln = Gd, Er) solid solutionPham, V. M.*; Arima, Tatsumi*; Inagaki, Yaohiro*; Idemitsu, Kazuya*; Akiyama, Daisuke*; Nagai, Takayuki; Okamoto, Yoshihiro
Journal of Nuclear Materials, 556, p.153189_1 - 153189_9, 2021/12
Times Cited Count:1 Percentile:15.7(Materials Science, Multidisciplinary)The crystal structure change was evaluated for (1-x)UO -xLnO
-xLnO (Ln=Gd or Er; x = 0 to 0.4) samples sintered at 1973 K for 8 h under Ar and Ar-10%H
(Ln=Gd or Er; x = 0 to 0.4) samples sintered at 1973 K for 8 h under Ar and Ar-10%H atmospheres. The effect of LnO
atmospheres. The effect of LnO doping on the crystal structure of UO
doping on the crystal structure of UO was investigated by X-ray diffraction (XRD) and X-ray absorption fine structure (XAFS). LnO
was investigated by X-ray diffraction (XRD) and X-ray absorption fine structure (XAFS). LnO doping into UO
doping into UO reduced the lattice parameter of UO
reduced the lattice parameter of UO -LnO
-LnO solid solutions up to 40mol% LnO
solid solutions up to 40mol% LnO . The lattice parameters of these samples were comparable to those of stoichiometric (U,Ln)O
. The lattice parameters of these samples were comparable to those of stoichiometric (U,Ln)O solid solutions, that is, the O/M ratios were close to 2.00. The U L
solid solutions, that is, the O/M ratios were close to 2.00. The U L -edge XANES analysis showed that higher U oxidation states of +5 or +6 formed, in addition to + 4. The EXAFS analysis indicated that the interatomic distances of U-O and Gd-O decreased with increasing x, whereas those of Er-O may not decrease monotonically.
-edge XANES analysis showed that higher U oxidation states of +5 or +6 formed, in addition to + 4. The EXAFS analysis indicated that the interatomic distances of U-O and Gd-O decreased with increasing x, whereas those of Er-O may not decrease monotonically.
Pham, V. H.; Kurata, Masaki; Steinbrueck, M.*
Thermo (Internet), 1(2), p.151 - 167, 2021/09
 I in mediterranean sea water
I in mediterranean sea waterPham, M. K.*; Betti, M.*; Povinec, P. P.*; Alfimov, V.*; Biddulph, D.*; Gastaud, J.*; Kieser, W. E.*; L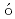 pez, Guti
pez, Guti rrez, J. M.*; Possnert, G.*; Sanchez-Cabeza, J. A.*; et al.
rrez, J. M.*; Possnert, G.*; Sanchez-Cabeza, J. A.*; et al.
Journal of Radioanalytical and Nuclear Chemistry, 286(1), p.121 - 127, 2010/10
Times Cited Count:14 Percentile:67.54(Chemistry, Analytical)no abstracts in English