Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
 - and K-montmorillonite
- and K-montmorilloniteKawakita, Ryohei; Saito, Akito*; Sakuma, Hiroshi*; Anraku, Sohtaro; Kikuchi, Ryosuke*; Otake, Tsubasa*; Sato, Tsutomu*
Applied Clay Science, 231, p.106722_1 - 106722_7, 2023/01
Times Cited Count:2 Percentile:17.27(Chemistry, Physical)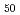 Cu
Cu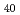 Al
Al bulk glassy alloy
bulk glassy alloyOnodera, Naoto*; Ishii, Akito*; Ishii, Koji*; Iwase, Akihiro*; Yokoyama, Yoshihiko*; Saito, Yuichi; Ishikawa, Norito; Yabuuchi, Atsushi*; Hori, Fuminobu*
Nuclear Instruments and Methods in Physics Research B, 314, p.122 - 124, 2013/11
Times Cited Count:3 Percentile:25.62(Instruments & Instrumentation)It has been reported that heavy ion irradiation causes softening in some cases of Zr-based bulk metallic glass alloys. However, the fundamental mechanisms of such softening have not been clarified yet. In this study, Zr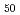 Cu
Cu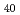 Al
Al bulk glassy alloys were irradiated with heavy ions of 10 MeV I at room temperature. Positron annihilation measurements have performed before and after irradiation to investigate changes in free volume. We discuss the relationship between the energy loss and local open volume change after 10 MeV I irradiation compared with those obtained for 200 MeV Xe and 5 MeV Al. The energy loss analysis in ion irradiation for the positron lifetime has revealed that the decreasing trend of positron lifetime is well expressed as a function of total electronic energy deposition rather than total elastic energy deposition. It means that the positron lifetime change by the irradiation has a relationship with the inelastic collisions with electrons during heavy ion irradiation.
bulk glassy alloys were irradiated with heavy ions of 10 MeV I at room temperature. Positron annihilation measurements have performed before and after irradiation to investigate changes in free volume. We discuss the relationship between the energy loss and local open volume change after 10 MeV I irradiation compared with those obtained for 200 MeV Xe and 5 MeV Al. The energy loss analysis in ion irradiation for the positron lifetime has revealed that the decreasing trend of positron lifetime is well expressed as a function of total electronic energy deposition rather than total elastic energy deposition. It means that the positron lifetime change by the irradiation has a relationship with the inelastic collisions with electrons during heavy ion irradiation.
Chiba, Go; Okumura, Keisuke; Oizumi, Akito*; Saito, Masaki*
Journal of Nuclear Science and Technology, 47(7), p.652 - 660, 2010/07
Times Cited Count:7 Percentile:43.22(Nuclear Science & Technology)In order to specify important nuclear data for accurate prediction of fission product concentrations(FPC), we extensively evaluate sensitivities of FPC to nuclear data with the depletion perturbation theory. The target fission products are twelve important ones for the burnup credit. The present study successfully specifies the important nuclear data both in a UO cell and in a MOX cell. While the obtained sensitivities are mostly similar to each other between the UO
cell and in a MOX cell. While the obtained sensitivities are mostly similar to each other between the UO and MOX cells, large differences are observed in some cases, such as the Gd-155 concentration. It is clearly shown that such differences between the UO
and MOX cells, large differences are observed in some cases, such as the Gd-155 concentration. It is clearly shown that such differences between the UO and MOX cells come from differences in cumulative fission yields between U-235 and Pu-239 and differences in neutron flux energy spectra.
and MOX cells come from differences in cumulative fission yields between U-235 and Pu-239 and differences in neutron flux energy spectra.
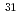 Ne
NeNakamura, Takashi*; Kobayashi, Nobuyuki*; Kondo, Yosuke*; Sato, Yoshiteru*; Aoi, Nori*; Baba, Hidetada*; Deguchi, Shigeki*; Fukuda, Naoki*; Gibelin, J.*; Inabe, Naoto*; et al.
Physical Review Letters, 103(26), p.262501_1 - 262501_4, 2009/12
Times Cited Count:213 Percentile:97.52(Physics, Multidisciplinary)no abstracts in English
Kubono, Shigeru*; Teranishi, Takashi*; Notani, Masahiro*; Yamaguchi, Hidetoshi*; Saito, Akito*; He, J. J.*; Wakabayashi, Yasuo*; Fujikawa, Hisashi*; Amadio, G.*; Baba, Hidetada*; et al.
Nuclear Physics A, 758, p.733 - 736, 2005/07
Times Cited Count:1 Percentile:14.20(Physics, Nuclear)With using 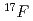 RNB from CRIB, proton inelastic scattering was observed. From this experiment, some resonance parameters have been deduced for the key reaction,
RNB from CRIB, proton inelastic scattering was observed. From this experiment, some resonance parameters have been deduced for the key reaction, 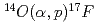 at the explosive hydrogen burning stage in stars. Proton inelastic scattering of
at the explosive hydrogen burning stage in stars. Proton inelastic scattering of 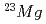 are also reported.
are also reported.
Saito, Akito*; Sakuma, Hiroshi*; Oda, Chie; Honda, Akira; Sato, Tsutomu*
no journal, ,
Adsorption of ammonium ion as a reducing product of nitrate on montmorillonite (Mt) may influence the suitable physical properties like expandability of the bentonite as the buffer material in geological disposal system for nitrates containing radioactive waste. Expandability and chemical stability of ammonium-Mt was studied by XRD measurement of the basal spacing and by molecular dynamics simulation. The experimental results and the simulations showed that (1) ammonium-Mt, unlike Mt with other types of interlayer cation, exhibited crystalline swelling with 1-layer hydrate structure under low relative humidity conditions, and this feature may be due to that mixing enthalpy in the ammonium-Mt and water system at low water content was negatively large compared with that of Mt with other types of interlayer cation, (2) the interlayer cation in Mt did not change from ammonium ion even after the interaction with ammonia water which ammonia (aq) was dominant species in, while (3) the interlayer ammonium ion was replaced by sodium ion by the interaction with sodium hydroxide solution.
Saito, Akito*; Kawakita, Ryohei*; Sakuma, Hiroshi*; Oda, Chie; Honda, Akira; Sato, Tsutomu*
no journal, ,
To understand the swelling behavior of NH -montmorillonite (MMT), the expandability of monoionic Na-, K- and NH
-montmorillonite (MMT), the expandability of monoionic Na-, K- and NH -MMT was investigated by XRD under controlled relative humidity (RH) conditions and molecular dynamics (MD) calculations. XRD results indicate that the expansion properties of NH
-MMT was investigated by XRD under controlled relative humidity (RH) conditions and molecular dynamics (MD) calculations. XRD results indicate that the expansion properties of NH -MMT are similar to that of K-MMT for RH
-MMT are similar to that of K-MMT for RH  30%. At RH
30%. At RH  30%, however, NH
30%, however, NH -MMT expands further and has a larger basal spacing than both Na- and K-MMT. MD calculation results showed that NH
-MMT expands further and has a larger basal spacing than both Na- and K-MMT. MD calculation results showed that NH -MMT reaches a one-layer hydration state under lower RH conditions than that of Na- and K-MMT, and was entirely consistent with the above observations by XRD. It was also confirmed that an irregular motion of interlayer NH
-MMT reaches a one-layer hydration state under lower RH conditions than that of Na- and K-MMT, and was entirely consistent with the above observations by XRD. It was also confirmed that an irregular motion of interlayer NH
 could result from bonds between H atoms of the NH
could result from bonds between H atoms of the NH
 ion and the base O atoms of MMT. This irregular motion enabled the basal spacing of NH
ion and the base O atoms of MMT. This irregular motion enabled the basal spacing of NH -MMT to increase and consequently, to expand further under low RH conditions.
-MMT to increase and consequently, to expand further under low RH conditions.
Kawakita, Ryohei*; Saito, Akito*; Sakuma, Hiroshi*; Anraku, Sohtaro; Oda, Chie; Mihara, Morihiro; Sato, Tsutomu*
no journal, ,
no abstracts in English
Kitano, Akihiro; Mori, Tetsuya; Nagata, Akito*; Saito, Kosuke; Misawa, Tsuyoshi*; Tamagawa, Yoichi*
no journal, ,
no abstracts in English
 Al
Al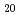
Tokunaga, Yo; Sakai, Hironori; Saito, Yo; Kambe, Shinsaku; Sakai, Akito*; Nakatsuji, Satoru*
no journal, ,
no abstracts in English
 -montmorillonite
-montmorilloniteKawakita, Ryohei*; Saito, Akito*; Sakuma, Hiroshi*; Anraku, Sohtaro; Oda, Chie; Mihara, Morihiro; Sato, Tsutomu*
no journal, ,
no abstracts in English
Saito, Akito*; Sakuma, Hiroshi*; Oda, Chie; Honda, Akira; Sato, Tsutomu*
no journal, ,
Chemical transformations of montmorillonite, a key component of bentonite, by ammonium ion as a reducing product of nitrate, may influence the suitable physical properties like expandability of the bentonite as the buffer material in deep geological disposal system for nitrates containing radioactive waste. Basal spacing of ammonium-montmorillonite before or after the interaction with 1M-ammonia water (pH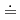 12) was studied by XRD. Molecular dynamics method was also used to simulate the behavior of cation and water in the interlayer of ammonium-montmorillonite. The experimental results suggested that the interlayer cation in montmorillonite did not change from ammonium ion even after the interaction with ammonia water (pH
12) was studied by XRD. Molecular dynamics method was also used to simulate the behavior of cation and water in the interlayer of ammonium-montmorillonite. The experimental results suggested that the interlayer cation in montmorillonite did not change from ammonium ion even after the interaction with ammonia water (pH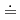 12) which ammonia(aq) was dominant species in, and crystalline swelling behavior of ammonium-montmorillonite differed from that of montmorillonite containing other type of interlayer cation. MD calculation indicated these features may be due to the hydrogen bond between ammonium ion and basal oxygen of montmorillonite.
12) which ammonia(aq) was dominant species in, and crystalline swelling behavior of ammonium-montmorillonite differed from that of montmorillonite containing other type of interlayer cation. MD calculation indicated these features may be due to the hydrogen bond between ammonium ion and basal oxygen of montmorillonite.
Fujitsu, Akito*; Odaira, Naoya*; Ito, Daisuke*; Ito, Kei*; Saito, Yasushi*; Imaizumi, Yuya; Matsuba, Kenichi; Kamiyama, Kenji
no journal, ,
no abstracts in English