Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
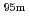 Tc for Compton camera imaging
Tc for Compton camera imagingHatsukawa, Yuichi; Hashimoto, Kazuyuki; Tsukada, Kazuaki; Sato, Tetsuya; Asai, Masato; Toyoshima, Atsushi; Nagai, Yasuki; Tanimori, Toru*; Sonoda, Shinya*; Kabuki, Shigeto*; et al.
Journal of Radioanalytical and Nuclear Chemistry, 303(2), p.1283 - 1285, 2015/02
Times Cited Count:2 Percentile:16.04(Chemistry, Analytical)Technetium-99m ( Tc) is used in radioactive medical diagonostic tests, for example as a radioactive tracer that medical equipment can detect in the human body. It is well suited to the role because it emits readily detectable 141 keV
Tc) is used in radioactive medical diagonostic tests, for example as a radioactive tracer that medical equipment can detect in the human body. It is well suited to the role because it emits readily detectable 141 keV  rays, and its half-life is 6.01 hours (meaning that about 94% of it decays to technetium-99 in 24 hours). There are at least 31 commonly used radiopharmaceuticals based on technetium-99m for imaging and functional studies of the brain, myocardium, thyroid, lungs, liver, gallbladder, kidneys, skeleton, blood, and tumors. Recent years, with the develop-ment of the Compton camera which can realize high position resolution, technetium isotopes emitting high energy
rays, and its half-life is 6.01 hours (meaning that about 94% of it decays to technetium-99 in 24 hours). There are at least 31 commonly used radiopharmaceuticals based on technetium-99m for imaging and functional studies of the brain, myocardium, thyroid, lungs, liver, gallbladder, kidneys, skeleton, blood, and tumors. Recent years, with the develop-ment of the Compton camera which can realize high position resolution, technetium isotopes emitting high energy  -rays are required. In this study, technetium-95m which emits some
-rays are required. In this study, technetium-95m which emits some  rays around 800 keV was produced by the
rays around 800 keV was produced by the 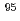 Mo(p,n)
Mo(p,n)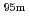 Tc reaction.
Tc reaction.
 Re-labeled antibody (A7) for radioimmunotherapy with rhenium(I) tricarbonyl core as a chelate site
Re-labeled antibody (A7) for radioimmunotherapy with rhenium(I) tricarbonyl core as a chelate siteOgawa, Kazuma*; Kawashima, Hidekazu*; Kinuya, Seigo*; Shiba, Kazuhiro*; Onoguchi, Masahisa*; Kimura, Hiroyuki*; Hashimoto, Kazuyuki; Odani, Akira*; Saji, Hideo*
Annals of Nuclear Medicine, 23(10), p.843 - 848, 2009/12
Times Cited Count:10 Percentile:32.81(Radiology, Nuclear Medicine & Medical Imaging)Rhenium is one of the most valuable elements for internal radiotherapy because  Re have favorable physical characteristics. However, there are problems when proteins such as antibodies are used as carriers of
Re have favorable physical characteristics. However, there are problems when proteins such as antibodies are used as carriers of  Re. Labeling methods require the complicated processes. Therefore, we planned the preparation by a simple method and evaluation of a stable
Re. Labeling methods require the complicated processes. Therefore, we planned the preparation by a simple method and evaluation of a stable  Re-labeled antibody. For this purpose, we selected
Re-labeled antibody. For this purpose, we selected  Re(I) tricarbonyl complex as a chelating site. A7 was used as a model protein.
Re(I) tricarbonyl complex as a chelating site. A7 was used as a model protein.  Re-labeled A7 was prepared by directly reacting a
Re-labeled A7 was prepared by directly reacting a  Re(I) tricarbonyl precursor with A7.
Re(I) tricarbonyl precursor with A7.  Re-(CO)
Re-(CO) -A7 were prepared with radiochemical yields of 23-28%. After purification,
-A7 were prepared with radiochemical yields of 23-28%. After purification,  Re-(CO)
Re-(CO) -A7 showed a radiochemical purity of over 95%. In biodistribution experiments,
-A7 showed a radiochemical purity of over 95%. In biodistribution experiments,  Re-labeled A7 showed high uptakes in the tumor.
Re-labeled A7 showed high uptakes in the tumor.
 Re-MAG3-conjugated bisphosphonate (
Re-MAG3-conjugated bisphosphonate ( Re-MAG3-HBP), an agent for treatment of painful bone metastases
Re-MAG3-HBP), an agent for treatment of painful bone metastasesOgawa, Kazuma*; Mukai, Takahiro*; Kawai, Keiichi*; Takamura, Norito*; Hanaoka, Hirofumi*; Hashimoto, Kazuyuki; Shiba, Kazuhiro*; Mori, Hirofumi*; Saji, Hideo*
European Journal of Nuclear Medicine and Molecular Imaging, 36(1), p.115 - 121, 2009/01
Times Cited Count:25 Percentile:59.11(Radiology, Nuclear Medicine & Medical Imaging)We have developed a  Re-MAG3 complex-conjugated bisphosphonate (
Re-MAG3 complex-conjugated bisphosphonate ( Re-MAG3-HBP) for the treatment of painful bone metastases. We assumed competitive inhibitors of protein binding to be useful for procuring a favorable biodistribution of
Re-MAG3-HBP) for the treatment of painful bone metastases. We assumed competitive inhibitors of protein binding to be useful for procuring a favorable biodistribution of  Re-MAG3-HBP because it has been reported that the concurrent administration of
Re-MAG3-HBP because it has been reported that the concurrent administration of  Tc-MAG3 and drugs with high affinity for serum protein enhanced total clearance and tissue distribution. The protein binding of
Tc-MAG3 and drugs with high affinity for serum protein enhanced total clearance and tissue distribution. The protein binding of  Re-MAG3-HBP in a human serum albumin solution was significantly decreased by the addition of ceftriaxone. In the biodistribution experiments, pretreatment with ceftriaxone enhanced the clearance of the radioactivity of
Re-MAG3-HBP in a human serum albumin solution was significantly decreased by the addition of ceftriaxone. In the biodistribution experiments, pretreatment with ceftriaxone enhanced the clearance of the radioactivity of  Re-MAG3-HBP in blood and nontarget tissues but had no effect on accumulation in bone. The findings suggested that the use of protein-binding competitive inhibitors would be effective in improving the pharmacokinetics of radiopharmaceuticals with high affinity for serum protein.
Re-MAG3-HBP in blood and nontarget tissues but had no effect on accumulation in bone. The findings suggested that the use of protein-binding competitive inhibitors would be effective in improving the pharmacokinetics of radiopharmaceuticals with high affinity for serum protein.
 Re-complex-conjugated bisphosphonate for the palliation of metastatic bone pain in an animal model
Re-complex-conjugated bisphosphonate for the palliation of metastatic bone pain in an animal modelOgawa, Kazuma*; Mukai, Takahiro*; Asano, Daigo*; Kawashima, Hidekazu*; Kinuya, Seigo*; Shiba, Kazuhiro*; Hashimoto, Kazuyuki; Mori, Hirofumi*; Saji, Hideo*
Journal of Nuclear Medicine, 48(1), p.122 - 127, 2007/01
We developed a highly stable rhenium-186 ( Re)-MAG3 complex-conjugated bisphosphonate, (
Re)-MAG3 complex-conjugated bisphosphonate, ( Re-MAG3-HBP), for the treatment of painful bone metastases. This agent showed a superior biodistribution as a bone-seeking agent in normal mice when compared with
Re-MAG3-HBP), for the treatment of painful bone metastases. This agent showed a superior biodistribution as a bone-seeking agent in normal mice when compared with  Re-HEDP. In this study, we evaluated the therapeutic effects of
Re-HEDP. In this study, we evaluated the therapeutic effects of  Re-MAG3-HBP using an animal model of bone metastasis. In the rats treated with
Re-MAG3-HBP using an animal model of bone metastasis. In the rats treated with  Re-HEDP, tumor growth was comparable to that in untreated rats. In contrast, when
Re-HEDP, tumor growth was comparable to that in untreated rats. In contrast, when  Re-MAG3-HBP was administered, tumor growth was significantly inhibited. Allodynia induced by bone metastasis was attenuated by treatment with
Re-MAG3-HBP was administered, tumor growth was significantly inhibited. Allodynia induced by bone metastasis was attenuated by treatment with  Re-MAG3-HBP or
Re-MAG3-HBP or  Re-HEDP, but
Re-HEDP, but  Re-MAG3-HBP tended to be more effective. These results indicate that
Re-MAG3-HBP tended to be more effective. These results indicate that  Re-MAG3-HBP could be useful as a therapeutic agent for the palliation of metastatic bone pain.
Re-MAG3-HBP could be useful as a therapeutic agent for the palliation of metastatic bone pain.
Ogawa, Kazuma*; Mukai, Takahiro*; Arano, Yasushi*; Otaka, Akira*; Ueda, Masashi*; Uehara, Tomoya*; Magata, Yasuhiro*; Hashimoto, Kazuyuki; Saji, Hideo*
Nuclear Medicine and Biology, 33(4), p.513 - 520, 2006/05
Times Cited Count:57 Percentile:80.60(Radiology, Nuclear Medicine & Medical Imaging)To develop a radiopharmaceutical for the palliation of painful bone metastases based on the concept of bifunctional radiopharmaceuticals, we synthesized a bisphosphonate derivative labeled with rhenium-186 ( Re) that contains a hydroxyl group at the central carbon of its bisphosphonate structure and attached a stable
Re) that contains a hydroxyl group at the central carbon of its bisphosphonate structure and attached a stable  Re-MAMA chelate to the amino group of a 4-amino-butylidene-bisphosphonate derivative,
Re-MAMA chelate to the amino group of a 4-amino-butylidene-bisphosphonate derivative,  Re-MAMA-HBP, and investigated the effect of a hydroxyl group at the central carbon of its bisphosphonate structure on the affinity for hydroxyapatite and biodistribution by conducting a comparative study with
Re-MAMA-HBP, and investigated the effect of a hydroxyl group at the central carbon of its bisphosphonate structure on the affinity for hydroxyapatite and biodistribution by conducting a comparative study with  Re-MAMA-BP.
Re-MAMA-BP.  Re-MAMA-HBP was prepared by a reaction with
Re-MAMA-HBP was prepared by a reaction with  ReO
ReO
 and SnCl
and SnCl in citrate buffer after the deprotection of trityl groups of Tr-MAMA-HBP. After reversed phase HPLC,
in citrate buffer after the deprotection of trityl groups of Tr-MAMA-HBP. After reversed phase HPLC,  Re-MAMA-HBP had a radiochemical purity of over 95 %. Compared with
Re-MAMA-HBP had a radiochemical purity of over 95 %. Compared with  Re-MAMA-BP,
Re-MAMA-BP,  Re-MAMA-HBP showed a greater affinity for hydroxyapatite beads in vitro and accumulated a significantly higher level in the femur in vivo. Thus, the introduction of a hydroxyl group into
Re-MAMA-HBP showed a greater affinity for hydroxyapatite beads in vitro and accumulated a significantly higher level in the femur in vivo. Thus, the introduction of a hydroxyl group into  Re complex-conjugated bisphosphonates would be effective in enhancing accumulation in bone. These findings provide useful information on the design of bone-seeking therapeutic radiopharmaceuticals.
Re complex-conjugated bisphosphonates would be effective in enhancing accumulation in bone. These findings provide useful information on the design of bone-seeking therapeutic radiopharmaceuticals.
Ogawa, Kazuma*; Mukai, Takahiro*; Arano, Yasushi*; Ono, Masahiro*; Hanaoka, Hirofumi*; Ishino, Seigo*; Hashimoto, Kazuyuki; Nishimura, Hiroshi*; Saji, Hideo*
Bioconjugate Chemistry, 16(4), p.751 - 757, 2005/07
Times Cited Count:64 Percentile:87.36(Biochemical Research Methods)Rhenium-186-1-hydroxyethylidene-1,1-diphosphonate ( Re-HEDP) has been used for the palliation of metastatic bone pain. Delayed blood clearance and high gastric uptake of radioactivity have been observed upon injection, due to the instability of
Re-HEDP) has been used for the palliation of metastatic bone pain. Delayed blood clearance and high gastric uptake of radioactivity have been observed upon injection, due to the instability of  Re-HEDP in vivo. In this study, on the basis of the concept of bifunctional radiopharmaceuticals, we designed a stable
Re-HEDP in vivo. In this study, on the basis of the concept of bifunctional radiopharmaceuticals, we designed a stable  Re-mercaptoacetylglycylglycylglycine (MAG3) complex-conjugated bisphosphonate (
Re-mercaptoacetylglycylglycylglycine (MAG3) complex-conjugated bisphosphonate ( Re-MAG3-HBP). After purification by HPLC,
Re-MAG3-HBP). After purification by HPLC,  Re-MAG3-HBP was synthesized with a radiochemical purity of over 95%. In biodistribution experiments, the radioactivity level of
Re-MAG3-HBP was synthesized with a radiochemical purity of over 95%. In biodistribution experiments, the radioactivity level of  Re-MAG3-HBP in bone was significantly higher than that of
Re-MAG3-HBP in bone was significantly higher than that of  Re-HEDP. Blood clearance of
Re-HEDP. Blood clearance of  Re-MAG3-HBP was faster than that of
Re-MAG3-HBP was faster than that of  Re-HEDP. In addition, the gastric accumulation of
Re-HEDP. In addition, the gastric accumulation of  Re-MAG3-HBP radioactivity was lower than that of
Re-MAG3-HBP radioactivity was lower than that of  Re-HEDP. In conclusion,
Re-HEDP. In conclusion,  Re-MAG3-HBP is expected to be a useful radiopharmaceutical for the palliation of metastatic bone pain.
Re-MAG3-HBP is expected to be a useful radiopharmaceutical for the palliation of metastatic bone pain.
Ogawa, Kazuma*; Mukai, Takahiro*; Arano, Yasushi*; Hanaoka, Hirofumi*; Hashimoto, Kazuyuki; Nishimura, Hiroshi*; Saji, Hideo*
Journal of Labelled Compounds and Radiopharmaceuticals, 47(11), p.753 - 761, 2004/11
Times Cited Count:31 Percentile:64.04(Biochemical Research Methods)no abstracts in English
Horiuchi, Kazuko*; Konno, Aya*; Ueda, Mayumi*; Fukuda, Yoko*; Nishio, Saori*; Hashimoto, Kazuyuki; Saji, Hideo*
European Journal of Nuclear Medicine and Molecular Imaging, 31(3), p.388 - 398, 2004/03
Times Cited Count:22 Percentile:50.74(Radiology, Nuclear Medicine & Medical Imaging)no abstracts in English
Mukai, Takahiro*; Ogawa, Kazuma*; Arano, Yasushi*; Ono, Masahiro*; Fujioka, Yasushi*; Izumo, Mishiroku; Konishi, Junji*; Saji, Hideo*
Journal of Labelled Compounds and Radiopharmaceuticals, 44(Suppl.1), p.S617 - S618, 2001/05
no abstracts in English
Ogawa, Kazuma*; Ono, Masahiro*; Fujioka, Yasushi*; Saji, Hideo*; Mukai, Takahiro*; Konishi, Junji*; Uehara, Tomoya*; Arano, Yasushi*; Onoma, Katsuyuki
Kaku Igaku, 37(5), P. 577, 2000/09
no abstracts in English
Kawashima, Hidekazu*; Hirasawa, Makoto*; Kimura, Hiroyuki*; Ono, Masahiro*; Hashimoto, Kazuyuki; Saji, Hideo*
no journal, ,
no abstracts in English
 Re-labeled biotin as a labeling agent for antibodies
Re-labeled biotin as a labeling agent for antibodiesHirasawa, Makoto*; Kawashima, Hidekazu*; Ogawa, Kazuma*; Kimura, Hiroyuki*; Ono, Masahiro*; Hashimoto, Kazuyuki; Saji, Hideo*
no journal, ,
no abstracts in English
 Re-labeled antibody (A7) by a simple method using rhenium(I) tricarbonyl complex
Re-labeled antibody (A7) by a simple method using rhenium(I) tricarbonyl complexOgawa, Kazuma*; Kawashima, Hidekazu*; Kinuya, Seigo*; Yoshimoto, Mitsuyoshi*; Shiba, Kazuhiro*; Kimura, Hiroyuki*; Hashimoto, Kazuyuki; Mori, Hirofumi*; Saji, Hideo*
no journal, ,
 Re is one of the most useful radionuclides for internal radiotherapy. However, there is a problem when protein such as antibody is used as a carrier of
Re is one of the most useful radionuclides for internal radiotherapy. However, there is a problem when protein such as antibody is used as a carrier of  Re. The labeling method using bifunctional chelating agents require the conjugation of
Re. The labeling method using bifunctional chelating agents require the conjugation of  Re-complex to protein after radiolabeling with the bifunctional chelating agent. Then, we planned the preparation of a stable
Re-complex to protein after radiolabeling with the bifunctional chelating agent. Then, we planned the preparation of a stable  Re-labeled protein by a simple method. A7 monoclonal antibody was labeled by reacting
Re-labeled protein by a simple method. A7 monoclonal antibody was labeled by reacting  Re(I) tricarbonyl precursor with A7 directly.
Re(I) tricarbonyl precursor with A7 directly.  Re labeled A7 was prepared with radiochemical yield of 23%. After purification,
Re labeled A7 was prepared with radiochemical yield of 23%. After purification,  Re labeled A7 showed radiochemical purity over 98%. After 24 hours of incubation, about 93% of
Re labeled A7 showed radiochemical purity over 98%. After 24 hours of incubation, about 93% of  Re-A7 remained intact, which indicates
Re-A7 remained intact, which indicates  Re-A7 is stable in vitro. In biodistribution experiment, 11.2% of the injected dose/g of
Re-A7 is stable in vitro. In biodistribution experiment, 11.2% of the injected dose/g of  Re-A7 accumulated in the tumor at 24 hours postinjection, and tumor to blood ratio was over 1.0 at the same time.
Re-A7 accumulated in the tumor at 24 hours postinjection, and tumor to blood ratio was over 1.0 at the same time.
Ogawa, Kazuma*; Mukai, Takahiro*; Kawai, Keiichi*; Takamura, Norito*; Hanaoka, Hirofumi*; Hashimoto, Kazuyuki; Saji, Hideo*
no journal, ,
Previously, based on the concept of bifunctional radiopharmaceuticals, we developed a highly stable Re-MAG3 complex conjugated bisphosphonate (
Re-MAG3 complex conjugated bisphosphonate ( Re-MAG3-HBP) for treatment of the painful bone metastases. We assumed that competitive inhibitors of protein binding might be useful for
Re-MAG3-HBP) for treatment of the painful bone metastases. We assumed that competitive inhibitors of protein binding might be useful for  Re-MAG3-HBP because it has been reported that the concurrent administration between
Re-MAG3-HBP because it has been reported that the concurrent administration between  Tc-MAG3 and drugs with high protein binding affinity produced competitive displacement at the specific binding sites and enhanced total clearance and tissue distribution. In this study, the displacement effect between
Tc-MAG3 and drugs with high protein binding affinity produced competitive displacement at the specific binding sites and enhanced total clearance and tissue distribution. In this study, the displacement effect between  Re-MAG3-HBP and several protein binding inhibitors were investigated. Effects of each competitive protein binding inhibitor were examined in in vitro human serum. Bucolome, ceftriaxione and cefazolin as displacers of human serum albumin (HSA) binding site I, and ibuprofen as a displacer of HSA binding site II were added to each serum before addition of
Re-MAG3-HBP and several protein binding inhibitors were investigated. Effects of each competitive protein binding inhibitor were examined in in vitro human serum. Bucolome, ceftriaxione and cefazolin as displacers of human serum albumin (HSA) binding site I, and ibuprofen as a displacer of HSA binding site II were added to each serum before addition of  Re-MAG3-HBP. As a result of in vitro study, the free fraction rate of
Re-MAG3-HBP. As a result of in vitro study, the free fraction rate of  Re-MAG3-HBP was significantly increased by the addition of ceftriaxone. In biodistribution studies, the loading ceftriaxone enhanced the clearance of radioactivity of
Re-MAG3-HBP was significantly increased by the addition of ceftriaxone. In biodistribution studies, the loading ceftriaxone enhanced the clearance of radioactivity of  Re-MAG3-HBP in blood and non-target tissues without the reduction of the bone accumulation. These findings suggested that the use of a protein binding competitive inhibitor would be effective for lowering the side effects of
Re-MAG3-HBP in blood and non-target tissues without the reduction of the bone accumulation. These findings suggested that the use of a protein binding competitive inhibitor would be effective for lowering the side effects of  Re-MAG3-HBP.
Re-MAG3-HBP.
 Re-labeled bisphosphonate in a rat model of bone metastasis
Re-labeled bisphosphonate in a rat model of bone metastasisOgawa, Kazuma*; Mukai, Takahiro*; Asano, Daigo*; Kawashima, Hidekazu*; Hashimoto, Kazuyuki; Shiba, Kazuhiro*; Mori, Hirofumi*; Saji, Hideo*
no journal, ,
no abstracts in English