Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
 O in the film on Fe oxidized at high temperature in air
O in the film on Fe oxidized at high temperature in airHaruna, Takumi*; Yamamoto, Tatsuya*; Miyairi, Yoji*; Shibata, Toshio*; Taniguchi, Naoki; Sakamaki, Keiko; Tachikawa, Hirokazu*
Zairyo To Kankyo, 64(5), p.201 - 206, 2015/05
Diffusion coefficients of D O in the films was determined in order to estimate corrosion rate of carbon steel for the overpack in ground water. Fe plates were heated to form oxide films. The films were characterized with XRD and SEM. After that, the specimen was contacted with D
O in the films was determined in order to estimate corrosion rate of carbon steel for the overpack in ground water. Fe plates were heated to form oxide films. The films were characterized with XRD and SEM. After that, the specimen was contacted with D O for 5184 ks, followed by subjected to TDS to obtain an amount of D
O for 5184 ks, followed by subjected to TDS to obtain an amount of D O absorbing into the film. As a result, single-layered film of Fe
O absorbing into the film. As a result, single-layered film of Fe O
O was formed at 573 and 723 K, and double-layered film of Fe
was formed at 573 and 723 K, and double-layered film of Fe O
O and Fe
and Fe O
O was formed at 873 K. It was found that an amount of D
was formed at 873 K. It was found that an amount of D O in the film correlated linearly with a square root of the absorption period, and that the amount was steady for a long period. From the results and Fick's second law, diffusion coefficients of D
O in the film correlated linearly with a square root of the absorption period, and that the amount was steady for a long period. From the results and Fick's second law, diffusion coefficients of D O was determined as 9.7
O was determined as 9.7 10
10 cm
cm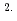 s
s for the Fe
for the Fe O
O film, and 5.5
film, and 5.5 10
10 cm
cm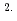 s
s to 2.2
to 2.2 10
10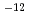 cm
cm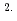 s
s for Fe
for Fe O
O film.
film.
Maeda, Toshikatsu; Watanabe, Koichi; Omori, Hiroyuki*; Sakamaki, Keiko; Inagaki, Yaohiro*; Idemitsu, Kazuya*
Genshiryoku Bakkuendo Kenkyu (CD-ROM), 21(2), p.63 - 74, 2014/12
Static leach tests were conducted for simulated HLW glass in CaCl /Ca(OH)
/Ca(OH) solutions to investigate the corrosion behavior of HLW glass under calcium-rich environments induced by cement based materials in geological repositories. Another series of leach tests were conducted in deionized water in the presence of iron to investigate the effects of iron over-pack on the glass corrosion. In Ca solutions, corrosion of the glass was inhibited during the test period compared to that in deionized water, while the corrosion was enhanced at the presence of iron. The enhancement of the glass corrosion was assumed to be accompanied with transformation of silica, a glass network former, into iron silicates.
solutions to investigate the corrosion behavior of HLW glass under calcium-rich environments induced by cement based materials in geological repositories. Another series of leach tests were conducted in deionized water in the presence of iron to investigate the effects of iron over-pack on the glass corrosion. In Ca solutions, corrosion of the glass was inhibited during the test period compared to that in deionized water, while the corrosion was enhanced at the presence of iron. The enhancement of the glass corrosion was assumed to be accompanied with transformation of silica, a glass network former, into iron silicates.
Sakamaki, Keiko; Kataoka, Masaharu; Maeda, Toshikatsu; Iida, Yoshihisa; Kamoshida, Michio; Yamaguchi, Tetsuji; Tanaka, Tadao
Corrosion Engineering, Science and Technology, 49(6), p.450 - 454, 2014/09
Times Cited Count:1 Percentile:0.00(Materials Science, Multidisciplinary)Corrosion experiments of a carbon steel plate embedded in bentonite mixture were conducted toverify our models assessing Eh evolution induced by corrosion of carbon steel overpack. Theexperimental results showed that the Eh decreased for the first 200 days and was subsequentlystabilised at around -450 mV; corrosion products were identified as magnetite and Fe waspresent mostly as divalent Fe within a 5 mm range from the carbon steel plate. Reactive transportmodelling was performed to assess the Eh evolution in the system using kinetic dissolution modelfor metallic iron and thermodynamic equilibrium models for other chemical reactions and closelyreproduced the experimental results. The models were verified only under the conditionsemployed in this study.
Sakamaki, Keiko
Genshiryoku Bakkuendo Kenkyu (CD-ROM), 20(2), p.109 - 110, 2013/12
no abstracts in English
Shibata, Toshio*; Taniguchi, Naoki; Sakamaki, Keiko; Tachikawa, Hirokazu*
no journal, ,
Carbon steel has been selected as a candidate material for the overpack and its corrosion behavior has been studied. The corrosion model of carbon steel in the oxygen depleted environment has been developed in case of a magnetite (Fe O
O ) corrosion film formed on the steel surface by assuming that diffusion of H
) corrosion film formed on the steel surface by assuming that diffusion of H O through the corrosion film controls the corrosion process. The improved model which includes the dissolution of the magnetite film has been proved successfully to predict the corrosion rate after a long exposure term. This study aims to simulate the corrosion behavior of carbon steel forming the siderite (FeCO
O through the corrosion film controls the corrosion process. The improved model which includes the dissolution of the magnetite film has been proved successfully to predict the corrosion rate after a long exposure term. This study aims to simulate the corrosion behavior of carbon steel forming the siderite (FeCO ) corrosion film and compare with the behavior of the magnetite film case. Based on the experimental results reported, the corrosion rate was estimated for two cases of 0.1 M and 0.25 mM of the total carbonate and compared with the experimental results. It was concluded that the siderite film formed more likely in the 0.1 M than 0.25 mM solution.
) corrosion film and compare with the behavior of the magnetite film case. Based on the experimental results reported, the corrosion rate was estimated for two cases of 0.1 M and 0.25 mM of the total carbonate and compared with the experimental results. It was concluded that the siderite film formed more likely in the 0.1 M than 0.25 mM solution.
Shimizu, Kosuke*; Inoue, Hiroyuki*; Taniguchi, Naoki; Sakamaki, Keiko; Tachikawa, Hirokazu*
no journal, ,
The effect of solution pH to the SCC susceptibility of carbon-steel (CS) in an anaerobic ground water must be investigated to guarantees the soundness of the CS HLW overpack for ultra-long term. For the present study, the SCC susceptibility of CS at and near the reversible hydrogen evolution potentials at each solution pH was evaluated by the slow strain rate test (SSRT); as for the test solution, NaHCO /Na
/Na CO
CO solutions whose pH were adjusted 8 to 13 and purged with Ar gas were used. The SCC susceptibility became minimum at pH near 9 and 13 as well as reached maximum at pH near 11.
solutions whose pH were adjusted 8 to 13 and purged with Ar gas were used. The SCC susceptibility became minimum at pH near 9 and 13 as well as reached maximum at pH near 11.
Sakamaki, Keiko; Otsuka, Ichiro*; Iida, Yoshihisa; Inada, Daisuke*; Kamoshida, Michio; Kataoka, Masaharu; Yamaguchi, Tetsuji; Tanaka, Tadao
no journal, ,
no abstracts in English
Yamaguchi, Tetsuji; Maeda, Toshikatsu; Mukai, Masayuki; Iida, Yoshihisa; Hemmi, Ko; Sawaguchi, Takuma; Sakamaki, Keiko
no journal, ,
no abstracts in English
Iwata, Hajime*; Sakamaki, Keiko*; Yasuda, Kenichiro; Onuki, Toshihiko; Utsunomiya, Satoshi*
no journal, ,
The aim of this study is to understand the fate of plutonium derived from atomic bomb, which has been preserved in the bottom sediment for 60 years, based on the investigation of the speciation and size distribution of plutonium. Approximately 60 % of plutonium is present as bound to organic matters and 30 % is present as insoluble species such as Pu oxides. Our results also suggest that the fast transformation of plutonium to the species bound to the organic matter for the period of 60 years, evidencing the important role of the natural organic matters on the plutonium speciation in the environment.
Sakamaki, Keiko; Iwata, Hajime*; Utsunomiya, Satoshi*
no journal, ,
no abstracts in English
Iwata, Hajime*; Kawamoto, Yuji*; Sakamaki, Keiko; Yasuda, Kenichiro; Onuki, Toshihiko; Utsunomiya, Satoshi*
no journal, ,
no abstracts in English
Maeda, Toshikatsu; Omori, Hiroyuki; Sakamaki, Keiko
no journal, ,
no abstracts in English