Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Akazawa, Daisuke; Sasaki, Takehiko*; Nagasaka, Masanari*; Shiga, Motoyuki
Journal of Chemical Physics, 156(4), p.044202_1 - 044202_7, 2022/01
Times Cited Count:3 Percentile:50.03(Chemistry, Physical)The hydration structure of cellulose is very important for understanding the hydrolysis of cellulose at the molecular level. In this paper, we report a joint experimental and theoretical study on the X-ray absorption spectroscopy (XAS) of aqueous cellobiose, a disaccharide unit of cellulose. In the experimental part, high resolution measurements of the carbon K-edge XAS spectra were performed. It was found that the peak heights in the spectrum change considerably over the temperature range of 25  C to 60
C to 60  C, which is a reflection of the number of hydrogen bonds between cellobiose and water. We suggest that this spectral change could be useful information for identifying the hydration of cellulose in various environments.
C, which is a reflection of the number of hydrogen bonds between cellobiose and water. We suggest that this spectral change could be useful information for identifying the hydration of cellulose in various environments.
Kondo, Tomomi; Sasaki, Takehiko*; Shiga, Motoyuki
Journal of Computational Chemistry, 42(25), p.1783 - 1791, 2021/09
Times Cited Count:1 Percentile:6.77(Chemistry, Multidisciplinary)Sugar alcohol dehydration in hot water is an important reaction that allows for environmentally friendly biomass conversion without the use of organic solvents. Here, we report a free-energy analysis by metadynamics (MTD) simulations based on ab initio and semiempirical electronic structure theory to understand the mechanism of dehydration reactions of sorbitol (SBT) in hot acidic water. It was found that the reaction proceeds via an S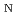 2 mechanism, whereby the free energy of protonation of the hydroxyl group created as an intermediate is affected by the acidic species. The free energy barriers of the reaction pathways leading to five-membered ether products are lower than those leading to six-membered ether products.
2 mechanism, whereby the free energy of protonation of the hydroxyl group created as an intermediate is affected by the acidic species. The free energy barriers of the reaction pathways leading to five-membered ether products are lower than those leading to six-membered ether products.
Kondo, Tomomi; Sasaki, Takehiko*; Ruiz-Barragan, S.*; Ribas-Ari o, J.*; Shiga, Motoyuki; Ruiz-Barragan, S.*
o, J.*; Shiga, Motoyuki; Ruiz-Barragan, S.*
Journal of Computational Chemistry, 42(3), p.156 - 165, 2021/01
Times Cited Count:4 Percentile:21.18(Chemistry, Multidisciplinary)We propose a canonical sampling method to refine metadynamics simulations a posteriori. This approach could be useful particularly when two or more free energy barriers are to be compared among chemical reactions in different or competing conditions. The method was then applied to study the acid dependence of polyalcohol dehydration reactions in high-temperature aqueous solutions. It was found that the reaction proceeds consistently via an S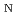 2 mechanism, whereby the free energy of protonation of the hydroxyl group created as an intermediate is affected significantly by the acidic species.
2 mechanism, whereby the free energy of protonation of the hydroxyl group created as an intermediate is affected significantly by the acidic species.
Chang, Y. L.*; Sasaki, Takehiko*; Ribas-Ari o, J.*; Machida, Masahiko; Shiga, Motoyuki
o, J.*; Machida, Masahiko; Shiga, Motoyuki
Journal of Physical Chemistry B, 123(7), p.1662 - 1671, 2019/02
Times Cited Count:4 Percentile:11.6(Chemistry, Physical)Dehydration of biomass-derived polyalcohols has recently drawn attention in green chemistry as a prototype of selective reactions controllable in hot water or hot carbonated water, without any use of organic solvents or metal catalysts. Here, we report a free-energy analysis based on first-principles metadynamics and blue-moon ensemble simulations to understand the mechanism of competing intramolecular dehydration reactions of 1,2,5-pentanetriol in hot acidic water. The simulations consistently predict that the most dominant mechanism is the proton-assisted S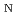 2 process, where the protonation of the hydroxyl group by water and the C-O bond breaking and formation occur in a single step. The detailed mechanism found from the simulations shows how the reaction paths are selective in hot water and why the reaction rates are accelerated in acidic environments, thus giving a clear explanation of experimental findings for a broad class of competing dehydration processes of polyalcohols.
2 process, where the protonation of the hydroxyl group by water and the C-O bond breaking and formation occur in a single step. The detailed mechanism found from the simulations shows how the reaction paths are selective in hot water and why the reaction rates are accelerated in acidic environments, thus giving a clear explanation of experimental findings for a broad class of competing dehydration processes of polyalcohols.
Kudo, Atsunari; Kurabayashi, Kazuaki; Yanagibashi, Futoshi; Sasaki, Shunichi; Sato, Takehiko; Fujimoto, Ikuo; Obu, Tomoyuki
Proceedings of 2017 International Congress on Advances in Nuclear Power Plants (ICAPP 2017) (CD-ROM), 6 Pages, 2017/04
The Co-processing process is the extraction process to recover Pu/U mixed product solution with given Pu/U ratio for improving of nuclear proliferation resistance. In addition, Np is also recovered with U and Pu because Np is one of minor actinides and a long-lived radionuclide and Np has the extractability into TBP solvent. Development of its flowsheet achieves to decrease environmental effect of waste materials. The orientation of development about Co-processing process is to demonstrate of reprocessing the future spent fuels from a LWR, a LWR-MOX hybrid, and a FR-MOX with one cycle. We demonstrated by use of miniature reflux-type centrifugal contactors at the partitioning unit. The test conditions of the Pu/U ratio in the loaded solvents were 1%, 3%, and 5% considering the composition of spent fuels. We used the HAN as the reductant of Np (VI) for back extraction. The results of these tests were very good. We got the prospect of U, Pu, and Np Co-processing flowsheet.
Ito, Takashi*; Wada, Seiichi*; Kakizaki, Takehiko; Kobayashi, Yasuhiko; Natsuhori, Masahiro*; Sano, Tadashi*; Sasaki, Nobuo*; Ito, Nobuhiko*
no journal, ,
no abstracts in English
Ito, Takashi*; Wada, Seiichi*; Kakizaki, Takehiko; Kobayashi, Yasuhiko; Natsuhori, Masahiro*; Sano, Tadashi*; Sasaki, Nobuo*; Ito, Nobuhiko*
no journal, ,
no abstracts in English
Hattori, Takanori; Arima, Hiroshi; Sano, Asami; Abe, Jun; Honda, Mitsunori; Fukazawa, Hiroshi; Utsumi, Wataru; Okuchi, Takuo*; Ono, Yoshiki*; Sasaki, Shigeo*; et al.
no journal, ,
The high-pressure neutron experiments above 10 GPa are limited so far due to the small neutron flux, which is insufficient for tiny high-pressure sample. Recent construction of the intense pulsed neutron source around the world has changed the situation. Inspired by the new Japanese pulsed neutron source JSNS at J-PARC, we started the high-P neutron experiments at the already operated beamlines (TAKUMI, NOVA) and the construction of the new beamline dedicated for high-pressure use (PLANET). This talk introduces these activities.