Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Koyama, Shinichi; Ikeuchi, Hirotomo; Mitsugi, Takeshi; Maeda, Koji; Sasaki, Shinji; Onishi, Takashi; Tsai, T.-H.; Takano, Masahide; Fukaya, Hiroyuki; Nakamura, Satoshi; et al.
Hairo, Osensui, Shorisui Taisaku Jigyo Jimukyoku Homu Peji (Internet), 216 Pages, 2023/11
In FY 2021 and 2022, JAEA perfomed the subsidy program for "the Project of Decommissioning and Contaminated Water Management (Development of Analysis and Estimation Technology for Characterization of Fuel Debris (Development of Technologies for Enhanced Analysis Accuracy, Thermal Bahavior Estimation, and Simplified Analysis of Fuel Debris)" started in FY 2021. This presentation material summarized the results of the project, which will be available shortly on the website of Management Office for the Project of Decommissioning, Contaminated Water and Treated Water Management.
Sasaki, Yuji; Nakase, Masahiko*; Kaneko, Masashi; Kobayashi, Toru; Takeshita, Kenji*; Matsumiya, Masahiko*
Analytical Sciences, 5 Pages, 2023/00
Times Cited Count:0 Percentile:0.00(Chemistry, Analytical)We conducted three field researches on Ru-extraction, XANES, and DFT-calculation. The order of the distribution ratio, D(Ru), from acid, HCl  H
H SO
SO
 HNO
HNO
 HClO
HClO , by MIDOA is studied by XANES spectra, which indicates the valency change of Ru in HCl media and supports the ion pairing extraction of anionic Ru ion and cationic MIDOA. The same extractant trend, NTAamide
, by MIDOA is studied by XANES spectra, which indicates the valency change of Ru in HCl media and supports the ion pairing extraction of anionic Ru ion and cationic MIDOA. The same extractant trend, NTAamide  MIDOA
MIDOA  IDOA, due to D values as the energy gap of HOMO and LUMO could be found by DFT calculation, which suggests that the reaction heat has a positive correlation with extractability for extractant.
IDOA, due to D values as the energy gap of HOMO and LUMO could be found by DFT calculation, which suggests that the reaction heat has a positive correlation with extractability for extractant.
 and Eu
and Eu ions onto sedimentary rock in the presence of gamma-irradiated humic acid
ions onto sedimentary rock in the presence of gamma-irradiated humic acidZhao, Q.*; Saito, Takeshi*; Miyakawa, Kazuya; Sasamoto, Hiroshi; Kobayashi, Taishi*; Sasaki, Takayuki*
Journal of Hazardous Materials, 428, p.128211_1 - 128211_10, 2022/04
Times Cited Count:7 Percentile:47.21(Engineering, Environmental)The influence of humic acid and its radiological degradation on the sorption of Cs and Eu
and Eu by sedimentary rock was investigated to understand the sorption process of metal ions and humic substances. Aldrich humic acid (HA) solution was irradiated with different doses of gamma irradiation using a Co-60 gamma-ray source prior to the contact between the metal ions and the solid sorbent. The HA molecule decomposed to smaller molecules with a lower complexation affinity. Batch sorption experiments were performed to evaluate the effect of gamma-irradiated HA on the sorption of Cs
by sedimentary rock was investigated to understand the sorption process of metal ions and humic substances. Aldrich humic acid (HA) solution was irradiated with different doses of gamma irradiation using a Co-60 gamma-ray source prior to the contact between the metal ions and the solid sorbent. The HA molecule decomposed to smaller molecules with a lower complexation affinity. Batch sorption experiments were performed to evaluate the effect of gamma-irradiated HA on the sorption of Cs and Eu
and Eu ions. The addition of non-irradiated HA weakened the sorption of Eu because of the lower sorption of the neutral or negatively charged Eu-HA complexes compared with free Eu ions. The sorption of monovalent Cs ions was barely affected by the presence of HA and its gamma irradiation. The concentration ratio of HA complexed species and non-complexed species in the solid and liquid phases was evaluated by sequential filtration and chemical equilibrium calculations. The ratios supported the minimal contribution of HA to Cs sorption. However, the concentration ratio for Eu
ions. The addition of non-irradiated HA weakened the sorption of Eu because of the lower sorption of the neutral or negatively charged Eu-HA complexes compared with free Eu ions. The sorption of monovalent Cs ions was barely affected by the presence of HA and its gamma irradiation. The concentration ratio of HA complexed species and non-complexed species in the solid and liquid phases was evaluated by sequential filtration and chemical equilibrium calculations. The ratios supported the minimal contribution of HA to Cs sorption. However, the concentration ratio for Eu in the liquid phase was high, indicating that the complexing ability of HA to Eu
in the liquid phase was high, indicating that the complexing ability of HA to Eu was higher than that of HA to Cs
was higher than that of HA to Cs ions. Therefore, the sorption of free Eu
ions. Therefore, the sorption of free Eu would predominate with the gamma irradiation dose applied to the HA solution under a radiation field near the HLW package.
would predominate with the gamma irradiation dose applied to the HA solution under a radiation field near the HLW package.
Nakayoshi, Akira; Mitsugi, Takeshi; Sasaki, Shinji; Maeda, Koji
JAEA-Data/Code 2021-011, 279 Pages, 2022/03
At the TEPCO's Fukushima Daiichi Nuclear Power Station (1F), an investigation inside the reactors has been carried out, and R&D has been made on methods of fuel debris retrieval and storage after retrieval. In order to carry out the decommissioning work safely and steadily, understanding characteristics of fuel debris in the reactors is required. Therefore, in the development of technologies for grasping and analyzing properties of fuel debris project, the characteristics of simulated fuel debris, such as hardness, drying behavior, etc., of fuel debris for design of removal and storage, have been investigated and estimated, and provided to other projects conducting the decommissioning work. As part of this project, U-containing particles in samples (e.g., deposit on the investigation equipment, sediment in the reactors, etc.) obtained during the internal investigation of the reactors of 1F units 1 to 3 were analyzed. This report summarized the results of FE-SEM/WDX, FE-SEM/EDS, STEM/EDS, and TEM analysis, which were extracted from all analysis results obtained, as a database for the evaluation of the generation mechanism of U-containing particles. The analyses were performed at the JAEA Oarai Research and Development Institute and Nippon Nuclear Fuel Development Co., LTD.
Sasaki, Yuji; Kaneko, Masashi; Ban, Yasutoshi; Matsumiya, Masahiko*; Nakase, Masahiko*; Takeshita, Kenji*
Separation Science and Technology, 57(16), p.2543 - 2553, 2022/00
Times Cited Count:4 Percentile:29.12(Chemistry, Multidisciplinary)The mutual separation of actinides (An) from lanthanides (Ln) using the masking agent of DTPA (diethylenetriamine-pentaacetic acid) or DTBA (diethylenetriamine-triacetic acid-bis(diethylacetamide)) in the aqueous phase through DGA extraction, referring TALSPEAK method, is focused. We investigate to obtain the same separation performance using commercially available DTPA on that using DTBA. In this work, we select lactic acid (LA) of pH buffer from 10 organic acids and ethylenediamine (ED) for the pH adjustment. Almost the same D and SF values are obtained among the conditions: TODGA-DTPA-LA-NaOH, TODGA-DTPA-LA-ED, and TODGA-DTBA-LA. The experimental results using batchwise multi-stage extractions show the average yields of Ln (La to Gd) and Am to be 3.73 and 98.1% in the aqueous phase using DGA-DTPA-LA-ED, to be 3.1 and 97.0% using DGA-DTPA-LA-NaOH, and to be 1.61 and 98.7% using DGA-DTBA-LA.
Sasaki, Yuji; Kaneko, Masashi; Matsumiya, Masahiko*; Nakase, Masahiko*; Takeshita, Kenji*
Solvent Extraction and Ion Exchange, 40(6), p.620 - 640, 2022/00
Times Cited Count:2 Percentile:13.50(Chemistry, Multidisciplinary)Owing to the chemical behavior of trivalent lanthanide and actinide ions with similar ionic radii, realizing this separation is still challenging. All lanthanides, Am, and Cm can be extracted using diglycolamide (DGA), and relatively high An/Ln separation efficiencies have been obtained using diethylenetriamine-triacetic-bisamide (DTBA). To improve the previous results as well as the separation conditions, we used organic acids for pH adjustment. The advantages of this modification included low HNO , DTBA concentrations and pH stability owing to the addition of lactic acid. Under these modified conditions, the recovery rates observed were as follows: 97.1% for Nd with the co-existence of 1.59% Am in organic phase, and 98.4% for Am with the co-existence of 2.95% Nd in aqueous phase.
, DTBA concentrations and pH stability owing to the addition of lactic acid. Under these modified conditions, the recovery rates observed were as follows: 97.1% for Nd with the co-existence of 1.59% Am in organic phase, and 98.4% for Am with the co-existence of 2.95% Nd in aqueous phase.
Kaneko, Masashi; Sasaki, Yuji; Wada, Eriko*; Nakase, Masahiko*; Takeshita, Kenji*
Chemistry Letters, 50(10), p.1765 - 1769, 2021/10
Times Cited Count:0 Percentile:0.00(Chemistry, Multidisciplinary)Density functional theory calculation is applied to predict the stability constants for Eu and Am
and Am complexes in aqueous solution for molecular modelling of novel separation agents for minor actinides over lanthanides. Logarithm of experimental stability constants correlates with calculated complex formation enthalpies with high reproducibility (R
complexes in aqueous solution for molecular modelling of novel separation agents for minor actinides over lanthanides. Logarithm of experimental stability constants correlates with calculated complex formation enthalpies with high reproducibility (R
 0.98). Prediction of stability constants of novel chelates is demonstrated and indicates a potential availability of the derivatives of diethylenetriaminepentaacetic acid type chelate in acidic condition and enhancement of Am
0.98). Prediction of stability constants of novel chelates is demonstrated and indicates a potential availability of the derivatives of diethylenetriaminepentaacetic acid type chelate in acidic condition and enhancement of Am selectivity over Eu
selectivity over Eu .
.
 /Eu
/Eu selectivity with diethylenetriaminepentaacetic acid and its bisamide chelates.
selectivity with diethylenetriaminepentaacetic acid and its bisamide chelates.Kaneko, Masashi; Sasaki, Yuji; Matsumiya, Masahiko*; Nakase, Masahiko*; Takeshita, Kenji*
Journal of Nuclear Science and Technology, 58(5), p.515 - 526, 2021/05
Times Cited Count:3 Percentile:27.12(Nuclear Science & Technology)Density-functional theory calculations were applied to molecular structure and complex formation reaction modelings of metal ion complexes with diethylenetriaminepentaacetic acid (DTPA) and its bisamide (DTPABA) chelates to understand the metal ions selectivity between Am and Eu
and Eu . The calculated complexes with DTPA and DTPABA chelates reproduced the coordination geometries of experimental crystal structures. Calculated Gibbs free energies of the complex formation reactions indicated that Am
. The calculated complexes with DTPA and DTPABA chelates reproduced the coordination geometries of experimental crystal structures. Calculated Gibbs free energies of the complex formation reactions indicated that Am ion forms higher stable complexes with both chelates than Eu
ion forms higher stable complexes with both chelates than Eu ion, being consistent with the experimental results. The higher Am
ion, being consistent with the experimental results. The higher Am selectivity over Eu
selectivity over Eu was suggested to originate in the larger bond overlap between Am
was suggested to originate in the larger bond overlap between Am 5f-orbital and N 2s, 2p-orbital. This mean that the covalent contribution between metal ion and donor atoms differentiates the complex formation stabilities, leading to the Am
5f-orbital and N 2s, 2p-orbital. This mean that the covalent contribution between metal ion and donor atoms differentiates the complex formation stabilities, leading to the Am /Eu
/Eu selectivity. We expect that this study contributes to systematize the origin of metal ions selectivity and to accelerate novel ligands exploration.
selectivity. We expect that this study contributes to systematize the origin of metal ions selectivity and to accelerate novel ligands exploration.
Matsuya, Yusuke; Kai, Takeshi; Sato, Tatsuhiko; Liamsuwan, T.*; Sasaki, Kohei*; Nikjoo, H.*
Physics in Medicine & Biology, 66(6), p.06NT02_1 - 06NT02_11, 2021/03
Times Cited Count:23 Percentile:87.23(Engineering, Biomedical)A general-purpose Monte Carlo radiation transport simulation code, Particle and Heavy Ion Transport code System (PHITS), has the ability to handle diverse particle types over a wide range of energy. In PHITS version 3.20, ion track structure mode has been developed based on the algorithms in the KURBUC code, which enables to simulate the atomic interactions by primary ion and secondary particles (named as PHITS-KURBUC mode). In this study, we compared the range, radial dose distributions, and microdosimetric distributions calculated using the PHITS-KURBUC mode to the corresponding data obtained from the original KURBUC and from other studies. These comparative studies confirm the successful inclusion of the KURBUC code in the PHITS code. As results of the synergistic effect between the macroscopic and microscopic radiation transport codes, this implementation enabled the detailed calculation of the microdosimetric and nanodosimetric quantities under complex radiation fields, such as proton beam therapy with the spread-out Bragg peak. This PHITS-KURBUC mode is expected to pave the way for next-generation radiation researches, such as radiation physics, radiological protection, medical physics, and radiation biology.
Takeda, Tetsuaki*; Inagaki, Yoshiyuki; Aihara, Jun; Aoki, Takeshi; Fujiwara, Yusuke; Fukaya, Yuji; Goto, Minoru; Ho, H. Q.; Iigaki, Kazuhiko; Imai, Yoshiyuki; et al.
High Temperature Gas-Cooled Reactors; JSME Series in Thermal and Nuclear Power Generation, Vol.5, 464 Pages, 2021/02
As a general overview of the research and development of a High Temperature Gas-cooled Reactor (HTGR) in JAEA, this book describes the achievements by the High Temperature Engineering Test Reactor (HTTR) on the designs, key component technologies such as fuel, reactor internals, high temperature components, etc., and operational experience such as rise-to-power tests, high temperature operation at 950 C, safety demonstration tests, etc. In addition, based on the knowledge of the HTTR, the development of designs and component technologies such as high performance fuel, helium gas turbine and hydrogen production by IS process for commercial HTGRs are described. These results are very useful for the future development of HTGRs. This book is published as one of a series of technical books on fossil fuel and nuclear energy systems by the Power Energy Systems Division of the Japan Society of Mechanical Engineers.
C, safety demonstration tests, etc. In addition, based on the knowledge of the HTTR, the development of designs and component technologies such as high performance fuel, helium gas turbine and hydrogen production by IS process for commercial HTGRs are described. These results are very useful for the future development of HTGRs. This book is published as one of a series of technical books on fossil fuel and nuclear energy systems by the Power Energy Systems Division of the Japan Society of Mechanical Engineers.
Matsumiya, Masahiko*; Tsuchida, Yusuke*; Sasaki, Yuji; Ono, Ryoma*; Nakase, Masahiko*; Takeshita, Kenji*
Journal of Radioanalytical and Nuclear Chemistry, 327(1), p.597 - 607, 2021/01
Times Cited Count:4 Percentile:35.32(Chemistry, Analytical)To achieve trichotomic separation of light lanthanides (Ln), heavy Ln, and Am, batchwise multi-stage extractions using tetraoctyl-diglycolamide (TODGA) extractant from organic acids are studied. Malonic acid (MA) has high solubility in water and is used as the main component of the aqueous phase. It is clear that the separation factor (SF) for Nd/Am from MA and that for La/Am from MA + HNO are both around 30. The light Ln (e.g., La and Ce) flowed-out in 1 M MA+0.05 M HNO
are both around 30. The light Ln (e.g., La and Ce) flowed-out in 1 M MA+0.05 M HNO (1st soln.), Am is recovered into 3 M MA (2nd soln.), and middle and heavy Ln (Nd and other heavy Ln) are back-extracted into 0.1 M TEDGA/water (3rd soln.). This extraction method can give 95% recovery of Am with total Ln of less than 16% present in high-level radioactive waste.
(1st soln.), Am is recovered into 3 M MA (2nd soln.), and middle and heavy Ln (Nd and other heavy Ln) are back-extracted into 0.1 M TEDGA/water (3rd soln.). This extraction method can give 95% recovery of Am with total Ln of less than 16% present in high-level radioactive waste.
 -tetraoctyl-diglycolamide from organic acid
-tetraoctyl-diglycolamide from organic acidSasaki, Yuji; Matsumiya, Masahiko*; Nakase, Masahiko*; Takeshita, Kenji*
Chemistry Letters, 49(10), p.1216 - 1219, 2020/10
Times Cited Count:9 Percentile:35.93(Chemistry, Multidisciplinary)Lanthanide (Ln) extractions from organic acids to  -dodecane by
-dodecane by  -tetraoctyl-diglycolamide (TODGA) were conducted. Four organic acids (lactic acid, malonic acid, tartaric acid, and citric acid) were employed. Although these acids stabilize lanthanides in the aqueous phase, a distribution ratio (
-tetraoctyl-diglycolamide (TODGA) were conducted. Four organic acids (lactic acid, malonic acid, tartaric acid, and citric acid) were employed. Although these acids stabilize lanthanides in the aqueous phase, a distribution ratio ( ) greater 1 was obtained for heavy Ln. Ln patterns (
) greater 1 was obtained for heavy Ln. Ln patterns ( (Ln) against atomic number of Ln) show maximum values of Ho and Er. In order to obtain high
(Ln) against atomic number of Ln) show maximum values of Ho and Er. In order to obtain high  values, the addition of HNO
values, the addition of HNO in aqueous phase is found to be effective.
in aqueous phase is found to be effective.
Ohashi, Hirofumi; Goto, Minoru; Ueta, Shohei; Sato, Hiroyuki; Fukaya, Yuji; Kasahara, Seiji; Sasaki, Koei; Mizuta, Naoki; Yan, X.; Aoki, Takeshi*
Proceedings of 9th International Topical Meeting on High Temperature Reactor Technology (HTR 2018) (USB Flash Drive), 6 Pages, 2018/10
Conceptual design study of an experimental HTGR is performed to upgrade the plant system from Japanese High Temperature engineering Test Reactor (HTTR) to a commercial HTGR. Safety systems of HTTR are upgraded to demonstrate the commercial HTGR concept, such as a passive reactor cavity cooling system, a confinement, etc. An intermediate heat exchanger (IHX) is replaced by a steam generator (SG) for a process heat supply to demonstrate the technology for a commercial use. This paper describes the conceptual design study results of the plant system of the experimental HTGR.
Kobayashi, Taishi*; Teshima, Takeshi*; Sasaki, Takayuki*; Kitamura, Akira
Journal of Nuclear Science and Technology, 54(2), p.233 - 241, 2017/02
Times Cited Count:9 Percentile:60.50(Nuclear Science & Technology)Zr solubility in the presence of gluconic acid (GLU) and isosaccharinic acid (ISA) was investigated as a function of hydrogen ion concentration (pH ) and the total concentration of GLU or ISA. The dependence of the increase in Zr solubility on the pH
) and the total concentration of GLU or ISA. The dependence of the increase in Zr solubility on the pH and GLU concentration suggested the existence of Zr(OH)
and GLU concentration suggested the existence of Zr(OH) (GLU)
(GLU)
 in the neutral pH region and Zr(OH)
in the neutral pH region and Zr(OH) (GLU)(GLU
(GLU)(GLU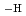 )
) in the alkaline pH region above pH
in the alkaline pH region above pH 10 as the dominant species in the presence of 10
10 as the dominant species in the presence of 10 - 10
- 10 mol/dm
mol/dm (M) GLU. In the presence of ISA, the dominant species Zr(OH)
(M) GLU. In the presence of ISA, the dominant species Zr(OH) (ISA)
(ISA)
 and Zr(OH)
and Zr(OH) (ISA)(ISA
(ISA)(ISA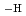 )
) were proposed to occur in the neutral and alkaline pH regions, similar to those found in the presence of GLU. From X-ray diffraction analysis, the solubility-limiting solid phase in the presence of GLU and ISA was considered to be Zr(OH)
were proposed to occur in the neutral and alkaline pH regions, similar to those found in the presence of GLU. From X-ray diffraction analysis, the solubility-limiting solid phase in the presence of GLU and ISA was considered to be Zr(OH) (am). The formation constants of the Zr gluconate and isosaccharinate complexes were determined by least squares fitting analysis of the solubility data, and the obtained values were discussed in comparison with those of tetravalent actinides.
(am). The formation constants of the Zr gluconate and isosaccharinate complexes were determined by least squares fitting analysis of the solubility data, and the obtained values were discussed in comparison with those of tetravalent actinides.
Sasaki, Akira; Nishihara, Katsunobu*; Sunahara, Atsushi*; Nishikawa, Takeshi*
Proceedings of SPIE, Vol.9776, p.97762C_1 - 97762C_6, 2016/03
Times Cited Count:1 Percentile:50.76(Optics)For the improvement of efficiency and output of the laser pumped plasma (LPP) extreme ultra-violet (EUV) light source, we present a hydrodynamics model of laser irradiated Sn targets. The model takes liquid/solid to gas transition and mixed phase condition of the flow into account for the calculation of the distribution of the particles produced by the pre-pulse laser irradiation and optimization of the EUV source. Firstly, we investigate the mechanisms of the fragmentation of the target and particle emission, including the effect of the equation of state of Sn, and secondly, an applicable model is proposed based on the analysis.
Sasaki, Yuji; Saeki, Morihisa; Sugo, Yumi; Ikeda, Yasuhisa*; Kawasaki, Takeshi*; Suzuki, Tomoya*; Ohashi, Akira*
Solvent Extraction Research and Development, Japan, 22(1), p.37 - 45, 2015/05
Times Cited Count:15 Percentile:42.12(Chemistry, Multidisciplinary)An extractant, methylimino-bis- -dioctylacetamide (MIDOA), was used for the extraction of soft acid metals. It was found that MIDOA can extract not only Cr(VI), Mo(VI), W(VI), Tc(VII) and Re(VII), whose metals can form the oxonium anions due to their high oxidation states, but also other metal cations, like Nb(V), Ta(V) and Pd(II). Analogous compounds, imino-bis-
-dioctylacetamide (MIDOA), was used for the extraction of soft acid metals. It was found that MIDOA can extract not only Cr(VI), Mo(VI), W(VI), Tc(VII) and Re(VII), whose metals can form the oxonium anions due to their high oxidation states, but also other metal cations, like Nb(V), Ta(V) and Pd(II). Analogous compounds, imino-bis- -dioctylacetamide (IDOA) and methylimino-bis-
-dioctylacetamide (IDOA) and methylimino-bis- -di-2-ethylhexylacetamide (MIDEHA), are synthesized and compared for their extractability. It is clear that these extractants have almost same or lower
-di-2-ethylhexylacetamide (MIDEHA), are synthesized and compared for their extractability. It is clear that these extractants have almost same or lower  values than those for MIDOA. In order to examine the effect on extractability with different donor atoms, TODGA (
values than those for MIDOA. In order to examine the effect on extractability with different donor atoms, TODGA ( -tetraoctyl-diglycolamide) and TDGA (
-tetraoctyl-diglycolamide) and TDGA ( -tetraoctyl-tyiodiglycolamide) having oxygen and sulfur donor are employed. The comparison of their extractabilities suggests that the trend of Pd and Re extraction is N
-tetraoctyl-tyiodiglycolamide) having oxygen and sulfur donor are employed. The comparison of their extractabilities suggests that the trend of Pd and Re extraction is N  S
S  O and N
O and N  O
O  S, respectively.
S, respectively.
Sasaki, Yuji; Tsubata, Yasuhiro; Kitatsuji, Yoshihiro; Sugo, Yumi; Shirasu, Noriko; Ikeda, Yasuhisa*; Kawasaki, Takeshi*; Suzuki, Tomoya*; Mimura, Hitoshi*; Usuda, Shigekazu*; et al.
JAEA-Research 2014-008, 220 Pages, 2014/06
The researches on Development of mutual separation technology of minor actinides by the novel hydrophilic and lipophilic diamide compounds, entrusted to Japan Atomic Energy Agency by the Ministry of Education, Culture, Sports, Science and Technology of Japan, from 2010 to 2012 are summarized. This project was composed of three themes, those are (1) Development of total recovery of MA+Ln: basic researches for new extractant, DOODA, (2) Development of mutual separation of Am/Cm/Ln: basic researches of Ln-complex, solvent extraction, and extraction chromatography, and (3) Evaluation of separation technique: process simulation. For topic (1), we summarized the information on characteristic of DOODA extractant. For topic (2), we summarized the information on structures of Ln-complexes, solvent extraction and chromatography. For topic (3), we summarized the information on conditions of mixer-settler and evaluation of each fraction separated.
Hasegawa, Noboru; Ochi, Yoshihiro; Kawachi, Tetsuya; Nishikino, Masaharu; Ishino, Masahiko; Imazono, Takashi; Kaihori, Takeshi; Morita, Toshimasa; Sasaki, Akira; Terakawa, Kota*; et al.
X-Ray Lasers 2012; Springer Proceedings in Physics, Vol.147, p.117 - 120, 2014/00
Times Cited Count:0 Percentile:0.00(Engineering, Electrical & Electronic)We have developed the femto-second laser pump and soft X-ray laser probe system in order to observe the dynamical processes of the femto-second laser ablation. By using this system, we succeed to obtain the temporal evolution of the soft X-ray reflectivity from the laser induced Pt surface. The results lead that the rate of decrease in the reflectivity of the probe beam has a non-linear relation with the pump laser fluence.
Katsuyama, Chie*; Nashimoto, Hiroaki*; Nagaosa, Kazuyo*; Ishibashi, Tomotaka*; Furuta, Kazuki*; Kinoshita, Takeshi*; Yoshikawa, Hideki; Aoki, Kazuhiro; Asano, Takahiro*; Sasaki, Yoshito; et al.
FEMS Microbiology Ecology, 86(3), p.532 - 543, 2013/12
Times Cited Count:15 Percentile:36.87(Microbiology)Anaerobic microbial activity has a major influence on the subsurface environment, and should be considered in subsurface activities including the construction of radioactive waste repositories. We investigated denitrification and methanogenesis in anoxic groundwater from 140 m depth in two boreholes, where the redox potential fluctuated. The average maximum potential denitrification rates, measured under anaerobic conditions in the two boreholes using an 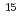 N tracer. Methanogenesis candidates were detected by 16S rRNA gene analysis. Although the stable isotope signatures suggested that some of the dissolved methane was of biogenic origin, no potential for methane production was evident during the incubations. The groundwater at 140 m depth did not contain oxygen, had an Eh ranging from -144 to 6.8 mV, and was found to be a potential field for denitrification.
N tracer. Methanogenesis candidates were detected by 16S rRNA gene analysis. Although the stable isotope signatures suggested that some of the dissolved methane was of biogenic origin, no potential for methane production was evident during the incubations. The groundwater at 140 m depth did not contain oxygen, had an Eh ranging from -144 to 6.8 mV, and was found to be a potential field for denitrification.
Onoda, Shinobu; Hasuike, Atsushi*; Nabeshima, Yoshiaki*; Sasaki, Hajime*; Yajima, Kotaro*; Sato, Shinichiro; Oshima, Takeshi
IEEE Transactions on Nuclear Science, 60(6), p.4446 - 4450, 2013/12
Times Cited Count:59 Percentile:96.81(Engineering, Electrical & Electronic)no abstracts in English