Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
 C
CPham, V. H.; Kurata, Masaki; Nagae, Yuji; Ishibashi, Ryo*; Sasaki, Masana*
Corrosion Science, 255, p.113098_1 - 113098_9, 2025/10
Times Cited Count:0Kokubun, Yuji; Hosomi, Kenji; Nagaoka, Mika; Seya, Natsumi; Inoue, Kazumi; Koike, Yuko; Uchiyama, Rei; Sasaki, Kazuki; Maehara, Yushi; Matsuo, Kazuki; et al.
JAEA-Review 2024-054, 168 Pages, 2025/03
The Nuclear Fuel Cycle Engineering Laboratories conducts environmental radiation monitoring around the reprocessing plant in accordance with the "Safety Regulations for Reprocessing Plant of JAEA, Part IV: Environmental Monitoring". This report summarizes the results of environmental radiation monitoring conducted during the period from April 2023 to March 2024 and the results of dose calculations for the surrounding public due to the release of radioactive materials from the plant into the atmosphere and ocean. In the results of the above environmental radiation monitoring, several items were affected by radioactive materials emitted from the accident at the Fukushima Daiichi Nuclear Power Station of Tokyo Electric Power Company, Incorporated (changed to Tokyo Electric Power Holdings, Inc. on April 1, 2016), which occurred in March 2011. In addition, environmental monitoring plan, analysis and measurement methods, monitoring data and their chronological change, meteorological data after statistical processing, status of radioactive waste release and evaluation results of the data over the normal range are included as appendices.
Sasaki, Yuji; Kaneko, Masashi; Ban, Yasutoshi; Suzuki, Tatsuya*
Solvent Extraction Research and Development, Japan, 32(1), p.21 - 29, 2025/00
The magnitude of the masking effect of carboxylic, amic-acidic, and amidic compounds through Ln and Am extractions by tetraoctyl diglycolamide (TODGA) is compared. The compounds used are diglycol, ethylenediamine, diethylenetriamine-type, and two other amides (dioxaoctane diamide and nitrylotriacetamide). The results show that below pH 1.2, where carboxylic acids are less dissociated, amide O atoms have higher reactivity with lanthanides than O atoms in carboxyl groups. From observing the Ln patterns (D(Ln) vs. their atomic number), the compounds primarily show high reactivity, with middle and heavy Ln having a higher charge density than light Ln. Four amide compounds are employed in this work. Those with tertiary amine N atoms have pH dependence on D(Ln) due to protonation and dissociation from amine N atoms.
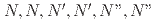 -hexahexyl-nitrilotriacetamide and electrodeposition
-hexahexyl-nitrilotriacetamide and electrodepositionMatsumiya, Masahiko*; Tokumitsu, Shun*; Mishima, Takumi*; Sasaki, Yuji
ECS Advances (Internet), 3(4), p.043001_1 - 043001_8, 2024/12
The extraction behavior of Rh(III) with Hexahexyl-nitrilotriacetamide, NTAamide(C6) was investigated in three different diluents (acetophenone, AP, 1,2-dichloroethane, DCE, and 1-octanol, OC). The electrochemical behavior of the extracted Rh(III) complex in each diluent was investigated from linear sweep voltammetry. It was revealed that Rh(III) was reduced to Rh(0) metal by a three electron transfer in NTAamide(C6)/AP, DCE and OC system. The electrodeposits can be recovered from continuous solvent extraction and direct electrodeposition. The electrodeposits were identified by XPS and XRD analyses as mainly Rh metal.
Tokumitsu, Shun*; Mishima, Takumi*; Matsumiya, Masahiko*; Sasaki, Yuji
Journal of Molecular Liquids, 414, Part A, p.126150_1 - 126150_8, 2024/11
The coordination states of Ln(III), (Ln=Pr, Nd, Tb and Dy) in ILs were investigated by Raman spectroscopy. The thermodynamic properties for the isomerism of [TFSA]- from trans- to cis-isomer were evaluated. The cis-[TFSA]- conformer bound to Ln3+ cation was the preferred coordination state of [Ln(III)(cis-TFSA)5]2-. The bonding energies of [Ln(III)(cis-TFSA)5]2-, (Ln=Pr, Nd, Tb and Dy) were estimated from DFT calculation.
 SO
SO and HF
and HFSasaki, Yuji; Kaneko, Masashi; Matsumiya, Masahiko*
Chemistry Letters, 53(9), p.upae164_1 - upae164_4, 2024/09
Times Cited Count:0 Percentile:0.00(Chemistry, Multidisciplinary)Ion-pair extractions enable to recover the anionic metal ions, such as TcO
 and ReO
and ReO
 , using cationic extractant. Recently the noble metals in hydrochloric acid are extracted by extractants having secondary and tertiary amino N atoms in their structures. Following this, extractions of Zr, Hf, Nb and Ta, metal anions present in sulfonic and hydrofluoric acids, are examined using this technique. Zr and Hf in H
, using cationic extractant. Recently the noble metals in hydrochloric acid are extracted by extractants having secondary and tertiary amino N atoms in their structures. Following this, extractions of Zr, Hf, Nb and Ta, metal anions present in sulfonic and hydrofluoric acids, are examined using this technique. Zr and Hf in H SO
SO , and Zr, Hf, Nb and Ta in HF can be extracted by NTAamide, MIDOA and TOA, and a basic information on their extraction behavior is obtained in this work.
, and Zr, Hf, Nb and Ta in HF can be extracted by NTAamide, MIDOA and TOA, and a basic information on their extraction behavior is obtained in this work.
Sasaki, Yuji; Kaneko, Masashi; Ban, Yasutoshi; Suzuki, Hideya*
Journal of Nuclear Science and Technology, 61(7), p.883 - 893, 2024/07
Times Cited Count:4 Percentile:59.85(Nuclear Science & Technology)The mutual separation of Am and Cm is conducted using an alkyl-diamide amine (ADAAM) extractant. ADAAM exhibits extremely high separation factor with respect to Am and Cm separation (5.9) in a nitric acid- -dodecane system. The batch-wise multistage extractions are performed using a system containing 0.2 M ADAAM and 1.5 M nitric acid. In this multistage extraction, an organic solvent give 96.5% and 1.06% yields of Am and Cm. After the mutual separation of Am and Cm, an additional extraction step is included to reduce the volumes of these aqueous and organic phases. Taking these steps, Am and Cm can be recovered in just two or three stages in the aqueous phases.
-dodecane system. The batch-wise multistage extractions are performed using a system containing 0.2 M ADAAM and 1.5 M nitric acid. In this multistage extraction, an organic solvent give 96.5% and 1.06% yields of Am and Cm. After the mutual separation of Am and Cm, an additional extraction step is included to reduce the volumes of these aqueous and organic phases. Taking these steps, Am and Cm can be recovered in just two or three stages in the aqueous phases.
Koyama, Shinichi; Ikeuchi, Hirotomo; Mitsugi, Takeshi; Maeda, Koji; Sasaki, Shinji; Onishi, Takashi; Tsai, T.-H.; Takano, Masahide; Fukaya, Hiroyuki; Nakamura, Satoshi; et al.
Hairo, Osensui, Shorisui Taisaku Jigyo Jimukyoku Homu Peji (Internet), 216 Pages, 2023/11
In FY 2021 and 2022, JAEA perfomed the subsidy program for "the Project of Decommissioning and Contaminated Water Management (Development of Analysis and Estimation Technology for Characterization of Fuel Debris (Development of Technologies for Enhanced Analysis Accuracy, Thermal Bahavior Estimation, and Simplified Analysis of Fuel Debris)" started in FY 2021. This presentation material summarized the results of the project, which will be available shortly on the website of Management Office for the Project of Decommissioning, Contaminated Water and Treated Water Management.
Kinoshita, Ryoma; Sasaki, Yuji; Kaneko, Masashi; Matsumiya, Masahiko*; Shinoku, Kota*; Shiroishi, Hidenobu*
Hydrometallurgy, 222, p.106159_1 - 106159_12, 2023/10
Times Cited Count:2 Percentile:23.52(Metallurgy & Metallurgical Engineering)Solvent extraction is conducted using a total of 20 metals revealing high stability constants with Cl and hexahexyl-nitrilotriacetamide (NTAamide(C6)) extractant. The metals used here may behave as anions at high Cl concentrations, and NTAamide(C6), which contains a tertiary N atom, is protonated under acidic conditions. Most of the metal ions in this study display higher distribution ratios (D(M)) from HCl than those from HNO , and exhibit 1:1 stoichiometries with NTAamide. Following the experimental results, the association constants and distribution coefficients of the group 12 elements are calculated via ion-pair extraction modeling using density functional theory calculations, and the simulations of D yield calculated values with the same trend as that of the measured values.
, and exhibit 1:1 stoichiometries with NTAamide. Following the experimental results, the association constants and distribution coefficients of the group 12 elements are calculated via ion-pair extraction modeling using density functional theory calculations, and the simulations of D yield calculated values with the same trend as that of the measured values.
Sasaki, Yuji; Kaneko, Masashi; Ban, Yasutoshi; Kinoshita, Ryoma; Matsumiya, Masahiko*; Shinoku, Kota*; Shiroishi, Hidenobu*
Analytical Sciences, 39(9), p.1575 - 1583, 2023/09
Times Cited Count:3 Percentile:35.90(Chemistry, Analytical)Extraction of Rh from HCl can be performed by NTAamide(C6) (hexahexyl-nitrilotriacetamide) and other related compounds into n-dodecane. We use ion-pair extraction of anionic species of Rh-chloride and protonated extractant. Rh behave as anion in hydrochloric acid and the tertiary nitrogen atom in extractant may be protonated to produce the quaternary amine in acidic condition. From the present work, the maximum distribution ratio of Rh(III) is 16. The D(Rh) values are changeable during preparation of the aqueous solutions because different Rh-Cl-H O complexes are formed in HCl media and show the slow exchange rate between Cl and H
O complexes are formed in HCl media and show the slow exchange rate between Cl and H O. Using the UV spectrum, Rh-chloride solution having the peak of spectrum at 504 nm can be extracted effectively, where RhCl
O. Using the UV spectrum, Rh-chloride solution having the peak of spectrum at 504 nm can be extracted effectively, where RhCl (H
(H O)
O) and RhCl
and RhCl (H
(H O)
O)
 exist mainly from DFT calculation. Stoichiometry of one-one complex of Rh and NTAamide is obtained from slope analysis, and 85 mM of concentrated Rh ion can be extracted.
exist mainly from DFT calculation. Stoichiometry of one-one complex of Rh and NTAamide is obtained from slope analysis, and 85 mM of concentrated Rh ion can be extracted.
 , Zr, and stainless steel and leaching behavior of the fission products and matrix elements
, Zr, and stainless steel and leaching behavior of the fission products and matrix elementsTonna, Ryutaro*; Sasaki, Takayuki*; Kodama, Yuji*; Kobayashi, Taishi*; Akiyama, Daisuke*; Kirishima, Akira*; Sato, Nobuaki*; Kumagai, Yuta; Kusaka, Ryoji; Watanabe, Masayuki
Nuclear Engineering and Technology, 55(4), p.1300 - 1309, 2023/04
Times Cited Count:6 Percentile:84.29(Nuclear Science & Technology)Simulated debris was synthesized using UO , Zr, and stainless steel and a heat treatment method under inert or oxidizing conditions. The primary U solid phase of the debris synthesized at 1473 K under inert conditions was UO
, Zr, and stainless steel and a heat treatment method under inert or oxidizing conditions. The primary U solid phase of the debris synthesized at 1473 K under inert conditions was UO , whereas a (U,Zr)O
, whereas a (U,Zr)O solid solution formed at 1873 K. Under oxidizing conditions, a mixture of U
solid solution formed at 1873 K. Under oxidizing conditions, a mixture of U O
O and (Fe,Cr)UO
and (Fe,Cr)UO phases formed at 1473 K whereas a (U,Zr)O
phases formed at 1473 K whereas a (U,Zr)O solid solution formed at 1873 K. The leaching behavior of the fission products from the simulated debris was evaluated using two methods: the irradiation method, for which fission products were produced via neutron irradiation, and the doping method, for which trace amounts of non-radioactive elements were doped into the debris. The dissolution behavior of U depended on the properties of the debris and aqueous medium the debris was immersed in. Cs, Sr, and Ba leached out regardless of the primary solid phases. The leaching of high-valence Eu and Ru ions was suppressed, possibly owing to their solid-solution reaction with or incorporation into the uranium compounds of the simulated debris.
solid solution formed at 1873 K. The leaching behavior of the fission products from the simulated debris was evaluated using two methods: the irradiation method, for which fission products were produced via neutron irradiation, and the doping method, for which trace amounts of non-radioactive elements were doped into the debris. The dissolution behavior of U depended on the properties of the debris and aqueous medium the debris was immersed in. Cs, Sr, and Ba leached out regardless of the primary solid phases. The leaching of high-valence Eu and Ru ions was suppressed, possibly owing to their solid-solution reaction with or incorporation into the uranium compounds of the simulated debris.
Sasaki, Yuji; Nakase, Masahiko*; Kaneko, Masashi; Kobayashi, Toru; Takeshita, Kenji*; Matsumiya, Masahiko*
Analytical Sciences, 5 Pages, 2023/00
Times Cited Count:0 Percentile:0.00(Chemistry, Analytical)We conducted three field researches on Ru-extraction, XANES, and DFT-calculation. The order of the distribution ratio, D(Ru), from acid, HCl  H
H SO
SO
 HNO
HNO
 HClO
HClO , by MIDOA is studied by XANES spectra, which indicates the valency change of Ru in HCl media and supports the ion pairing extraction of anionic Ru ion and cationic MIDOA. The same extractant trend, NTAamide
, by MIDOA is studied by XANES spectra, which indicates the valency change of Ru in HCl media and supports the ion pairing extraction of anionic Ru ion and cationic MIDOA. The same extractant trend, NTAamide  MIDOA
MIDOA  IDOA, due to D values as the energy gap of HOMO and LUMO could be found by DFT calculation, which suggests that the reaction heat has a positive correlation with extractability for extractant.
IDOA, due to D values as the energy gap of HOMO and LUMO could be found by DFT calculation, which suggests that the reaction heat has a positive correlation with extractability for extractant.
Simonnet, M.; Sasaki, Yuji; Yaita, Tsuyoshi
Solvent Extraction and Ion Exchange, 41(7), p.857 - 867, 2023/00
Times Cited Count:4 Percentile:41.44(Chemistry, Multidisciplinary)Fukaya, Yuji; Okita, Shoichiro; Sasaki, Koei; Ueta, Shohei; Goto, Minoru; Ohashi, Hirofumi; Yan, X.
Nuclear Engineering and Design, 399, p.112033_1 - 112033_9, 2022/12
Times Cited Count:0 Percentile:0.00(Nuclear Science & Technology)Kernel migration of TRi-structural ISOtropic (TRISO) fuel for High Temperature Gas-cooled Reactor (HTGR) has been analyzed to investigate the potential dominating effects. Kernel migration is a major fuel failure mode and dominant to determine the lifetime of the fuel for High Temperature engineering Test Reactor (HTTR). However, this study shows that the result and reliability depend on the evaluation method. The evaluation method used in this study takes into account of actual distribution of Coated Fuel Particles (CFPs) and the resulting heterogeneous fuel temperature calculation with such distribution. The result shows that the Kernel Migration Rate (KMR) is predicted to be about 10% less compared with the most conservative evaluation.
Ohshima, Hiroyuki; Morishita, Masaki*; Aizawa, Kosuke; Ando, Masanori; Ashida, Takashi; Chikazawa, Yoshitaka; Doda, Norihiro; Enuma, Yasuhiro; Ezure, Toshiki; Fukano, Yoshitaka; et al.
Sodium-cooled Fast Reactors; JSME Series in Thermal and Nuclear Power Generation, Vol.3, 631 Pages, 2022/07
This book is a collection of the past experience of design, construction, and operation of two reactors, the latest knowledge and technology for SFR designs, and the future prospects of SFR development in Japan. It is intended to provide the perspective and the relevant knowledge to enable readers to become more familiar with SFR technology.
Sasaki, Yuji
Bunri Gijutsu, 52(2), p.103 - 107, 2022/03
We develop all-inclusive partitioning method for actinides and fission products in high-level radioactive waste. This process is based on the sequential solvent extraction. In order to recover Cs and Sr for the management by interim storage, crown ether compounds are employed. For the removal of Pd and Mo due to production of a stable vitrified object, methylimino-dioctylacetamide (MIDOA) is taken as an extractant. DGA can extract both actinides and trivalent lanthanides. In order to separate each other, dietylenetriamine-triacetic-diamide (DTBA) for the stripping reagent of MA. For the mutual separation of Am/Cm, DGA and DOODA extraction system is taken into consideration.
Nomizu, Daiki; Sasaki, Yuji; Kaneko, Masashi; Matsumiya, Masahiko*; Katsuta, Shoichi*
Journal of Radioanalytical and Nuclear Chemistry, 331(3), p.1483 - 1493, 2022/03
Times Cited Count:7 Percentile:73.20(Chemistry, Analytical)We studied the successive formation of water soluble DGA (diglycolamide) and DOODA (dioxaoctanediamide) for the mutual separation of Ln in this extraction system. TODGA (tetraoctyl-diglycolamide) and DOODA(C8) (tetraoctyl-dioxaoctanediamide) have the opposite trend to extract light and heavy Ln through Ln-patterns. Metal-complexes of two folding Ln ions with water-soluble DOODA and three folding with DGA are found and their observed formation constants are calculated. The suitable separation condition (aqueous phase: 30 mM DOODA(C2) in 1 M nitric acid, organic phase: 0.1 M TODGA in n-dodecane) of multi-stage extraction (10  10) is conducted. From the present work, it is clear that La, Pr and Nd are mainly present in aqueous phase, instead Sm-Dy exist in the organic phase.
10) is conducted. From the present work, it is clear that La, Pr and Nd are mainly present in aqueous phase, instead Sm-Dy exist in the organic phase.
Sasaki, Yuji; Kaneko, Masashi; Ban, Yasutoshi; Matsumiya, Masahiko*; Nakase, Masahiko*; Takeshita, Kenji*
Separation Science and Technology, 57(16), p.2543 - 2553, 2022/00
Times Cited Count:5 Percentile:28.02(Chemistry, Multidisciplinary)The mutual separation of actinides (An) from lanthanides (Ln) using the masking agent of DTPA (diethylenetriamine-pentaacetic acid) or DTBA (diethylenetriamine-triacetic acid-bis(diethylacetamide)) in the aqueous phase through DGA extraction, referring TALSPEAK method, is focused. We investigate to obtain the same separation performance using commercially available DTPA on that using DTBA. In this work, we select lactic acid (LA) of pH buffer from 10 organic acids and ethylenediamine (ED) for the pH adjustment. Almost the same D and SF values are obtained among the conditions: TODGA-DTPA-LA-NaOH, TODGA-DTPA-LA-ED, and TODGA-DTBA-LA. The experimental results using batchwise multi-stage extractions show the average yields of Ln (La to Gd) and Am to be 3.73 and 98.1% in the aqueous phase using DGA-DTPA-LA-ED, to be 3.1 and 97.0% using DGA-DTPA-LA-NaOH, and to be 1.61 and 98.7% using DGA-DTBA-LA.
Sasaki, Yuji; Kaneko, Masashi; Matsumiya, Masahiko*; Nakase, Masahiko*; Takeshita, Kenji*
Solvent Extraction and Ion Exchange, 40(6), p.620 - 640, 2022/00
Times Cited Count:2 Percentile:12.88(Chemistry, Multidisciplinary)Owing to the chemical behavior of trivalent lanthanide and actinide ions with similar ionic radii, realizing this separation is still challenging. All lanthanides, Am, and Cm can be extracted using diglycolamide (DGA), and relatively high An/Ln separation efficiencies have been obtained using diethylenetriamine-triacetic-bisamide (DTBA). To improve the previous results as well as the separation conditions, we used organic acids for pH adjustment. The advantages of this modification included low HNO , DTBA concentrations and pH stability owing to the addition of lactic acid. Under these modified conditions, the recovery rates observed were as follows: 97.1% for Nd with the co-existence of 1.59% Am in organic phase, and 98.4% for Am with the co-existence of 2.95% Nd in aqueous phase.
, DTBA concentrations and pH stability owing to the addition of lactic acid. Under these modified conditions, the recovery rates observed were as follows: 97.1% for Nd with the co-existence of 1.59% Am in organic phase, and 98.4% for Am with the co-existence of 2.95% Nd in aqueous phase.
Matsutani, Takafumi; Sasaki, Yuji; Katsuta, Shoichi*
Analytical Sciences, 37(11), p.1603 - 1609, 2021/11
Times Cited Count:7 Percentile:37.70(Chemistry, Analytical)We investigated the chemical behavior of lanthanides (Ln) using diglycolamide extractant with multistage extraction. We obtained the breakthrough curves for light and middle Ln. Our study reveals that the metal extraction limit depends on their  values and metal concentrations used in the experiments. From the multistage extractions of 15 aqueous phases and 15 organic phases, three curves (extraction curves, back-extraction curves, and separation curves) were obtained by changing the nitric acid concentration. As an example, under a condition of the separation curve experiment (aqueous phase: 0.5 M HNO
values and metal concentrations used in the experiments. From the multistage extractions of 15 aqueous phases and 15 organic phases, three curves (extraction curves, back-extraction curves, and separation curves) were obtained by changing the nitric acid concentration. As an example, under a condition of the separation curve experiment (aqueous phase: 0.5 M HNO , organic phase: 0.1 M TDDGA (
, organic phase: 0.1 M TDDGA ( -tetradecyl-diglycolamide) in
-tetradecyl-diglycolamide) in  -dodecane), a recovery of more than 99% of Sm in the organic phase with less than 1% Nd can be obtained.
-dodecane), a recovery of more than 99% of Sm in the organic phase with less than 1% Nd can be obtained.