Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
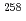 Md produced in the
Md produced in the  He +
He + 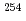 Es reaction
Es reactionNishio, Katsuhisa; Hirose, Kentaro; Makii, Hiroyuki; Orlandi, R.; Kean, K. R.*; Tsukada, Kazuaki; Toyoshima, Atsushi*; Asai, Masato; Sato, Tetsuya; Chiera, N. M.*; et al.
Physical Review C, 111(4), p.044609_1 - 044609_12, 2025/04
Times Cited Count:0 Percentile:0.00(Physics, Nuclear) He cryostat and a closed-cycle
He cryostat and a closed-cycle  He gas handling system installed in a SQUID magnetometer without continuous-cooling functionality
He gas handling system installed in a SQUID magnetometer without continuous-cooling functionalityShimamura, Kazutoshi*; Wajima, Hiroki*; Makino, Hayato*; Abe, Satoshi*; Haga, Yoshinori; Sato, Yoshiaki*; Kawae, Tatsuya*; Yoshida, Yasuo*
Japanese Journal of Applied Physics, 61(5), p.056502_1 - 056502_7, 2022/05
Times Cited Count:3 Percentile:24.69(Physics, Applied)G tz, M.*; Yakushev, A.*; G
tz, M.*; Yakushev, A.*; G tz, S.*; Di Nitto, A.*; D
tz, S.*; Di Nitto, A.*; D llmann, Ch. E.*; Asai, Masato; Kindler, B.*; Krier, J.*; Lommel, B.*; Nagame, Yuichiro*; et al.
llmann, Ch. E.*; Asai, Masato; Kindler, B.*; Krier, J.*; Lommel, B.*; Nagame, Yuichiro*; et al.
Radiochimica Acta, 110(2), p.75 - 86, 2022/02
Times Cited Count:2 Percentile:20.20(Chemistry, Inorganic & Nuclear)The study of volatile superheavy element carbonyl complexes requires more efficient methods because the yield of transactinide elements decreases with increasing atomic number. This is achieved by using a newly developed double chamber system to separate the recoil chamber and the reaction one, thereby avoiding the decomposition of reactive molecules by the projectile ion beam, which hinders the synthesis of carbonyl complexes. The feasibility of this method was verified by synthesizing 5d metal short-lived isotopes as homologous element isotopes of the light transactinide elements Sg, Bh, Hs, and Mt at the Japan Atomic Energy Agency tandem accelerator and conducting model experiments.

Chiera, N. M.*; Sato, Tetsuya; Eichler, R.*; Tomitsuka, Tomohiro; Asai, Masato; Adachi, Sadia*; Dressler, R.*; Hirose, Kentaro; Inoue, Hiroki*; Ito, Yuta; et al.
Angewandte Chemie; International Edition, 60(33), p.17871 - 17874, 2021/08
Times Cited Count:5 Percentile:24.90(Chemistry, Multidisciplinary)The formation and the chemical characterization of single atoms of dubnium (Db, element 105), in the form of its volatile oxychloride, was investigated using the on-line gas phase chromatography technique, in the temperature range 350 - 600  C. Under the exact same chemical conditions, comparative studies with the lighter homologs of group-5 in the Periodic Table clearly indicate the volatility sequence being NbOCl
C. Under the exact same chemical conditions, comparative studies with the lighter homologs of group-5 in the Periodic Table clearly indicate the volatility sequence being NbOCl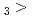 TaOCl
TaOCl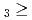 DbOCl
DbOCl . From the obtained experimental results, thermochemical data for DbOCl
. From the obtained experimental results, thermochemical data for DbOCl were derived. The present study delivers reliable experimental information for theoretical calculations on the chemical properties of transactinides.
were derived. The present study delivers reliable experimental information for theoretical calculations on the chemical properties of transactinides.
Chiera, N. M.; Sato, Tetsuya; Tomitsuka, Tomohiro; Asai, Masato; Ito, Yuta; Shirai, Kaori*; Suzuki, Hayato; Tokoi, Katsuyuki; Toyoshima, Atsushi; Tsukada, Kazuaki; et al.
Journal of Radioanalytical and Nuclear Chemistry, 320(3), p.633 - 642, 2019/06
Times Cited Count:2 Percentile:17.25(Chemistry, Analytical)An isothermal gas-chromatographic (IGC) device has been developed and tested for on-line gas phase studies of volatile oxychlorides of short-lived group-5 transition metals. Radioisotopes of niobium and tantalum, produced in nuclear fusion evaporation reactions, are directly flushed into the IGC setup by an inert gas-jet. Oxychloride compounds are formed by the addition of SOCl and O
and O . Parameters influencing the formation and transport of NbOCl
. Parameters influencing the formation and transport of NbOCl and TaOCl
and TaOCl are investigated. For nuclides with half-lives (
are investigated. For nuclides with half-lives (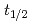 ) of about 30 s, an overall efficiency of 7% is obtained, rendering the IGC setup suitable for the chemical exploration of
) of about 30 s, an overall efficiency of 7% is obtained, rendering the IGC setup suitable for the chemical exploration of  Db(
Db(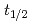 = 34s).
= 34s).
Chiera, N. M.; Sato, Tetsuya; Tomitsuka, Tomohiro; Asai, Masato; Suzuki, Hayato*; Tokoi, Katsuyuki; Toyoshima, Atsushi; Tsukada, Kazuaki; Nagame, Yuichiro
Inorganica Chimica Acta, 486, p.361 - 366, 2019/02
Times Cited Count:4 Percentile:20.64(Chemistry, Inorganic & Nuclear)The formation of NbOCl and TaOCl
and TaOCl and their adsorption behavior on quartz surfaces was explored by applying an isothermal gas-chromatographic method. Trace amounts of short-lived Nb and Ta isotopes were used. Adsorption enthalpy values (
and their adsorption behavior on quartz surfaces was explored by applying an isothermal gas-chromatographic method. Trace amounts of short-lived Nb and Ta isotopes were used. Adsorption enthalpy values ( ) at zero surface coverage of -
) at zero surface coverage of - (NbOCl
(NbOCl ) = 102
) = 102  4 kJ/mol and -
4 kJ/mol and - (TaOCl
(TaOCl ) = 128
) = 128  5 kJ/mol were determined by analyzing the chromatographic behavior of the Nb andTa complexes with a Monte-Carlo simulation method based on an adsorption-desorption kinetic model.By applying an empirical correlation, the experimental
5 kJ/mol were determined by analyzing the chromatographic behavior of the Nb andTa complexes with a Monte-Carlo simulation method based on an adsorption-desorption kinetic model.By applying an empirical correlation, the experimental  values were successively related to the macroscopic standard sublimation enthalpy,
values were successively related to the macroscopic standard sublimation enthalpy, 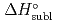 , as a measure of the volatility of each substance. The inferred sublimation enthalpies are in agreement with tabulated thermochemical values. Thus, the linear empirical correlation between
, as a measure of the volatility of each substance. The inferred sublimation enthalpies are in agreement with tabulated thermochemical values. Thus, the linear empirical correlation between  and
and 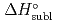 for metal-oxychlorides was updated with the inclusion of the present data. According to the predicted
for metal-oxychlorides was updated with the inclusion of the present data. According to the predicted 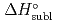 (DbOCl
(DbOCl ), a
), a  (DbOCl
(DbOCl ) value of 135
) value of 135  2 kJ/mol was extrapolated. The future accomplishment of comparative studies with DbOCl
2 kJ/mol was extrapolated. The future accomplishment of comparative studies with DbOCl under the same experimental conditions will provide valuable information on the volatility trend in Group-5 elements, together with an indication on the magnitude of relativistic effects on the electronic structure of dubnium.
under the same experimental conditions will provide valuable information on the volatility trend in Group-5 elements, together with an indication on the magnitude of relativistic effects on the electronic structure of dubnium.
Toyoshima, Atsushi; Kasamatsu, Yoshitaka*; Tsukada, Kazuaki; Asai, Masato; Ishii, Yasuo; Tome, Hayato*; Nishinaka, Ichiro; Sato, Tetsuya; Nagame, Yuichiro; Sch del, M.; et al.
del, M.; et al.
Journal of Nuclear and Radiochemical Sciences, 11(1), p.7 - 11, 2010/06
The extraction behavior of rutherfordium (Rf) into trioctylphosphine oxide (TOPO) from 2.0 - 7.0 M HCl solution was studied together with that of the homologues Zr and Hf. The extracted yields of Rf, Zr, and Hf increased with an increase of HCl concentration, and the sequence of their extraction was Zr  Hf
Hf  Rf. It is suggested that the stability of the RfCl
Rf. It is suggested that the stability of the RfCl
 2(TOPO) complex is lower than that of the corresponding species of the homologues.
2(TOPO) complex is lower than that of the corresponding species of the homologues.
Kasamatsu, Yoshitaka*; Toyoshima, Atsushi; Asai, Masato; Tsukada, Kazuaki; Li, Z.; Ishii, Yasuo; Tome, Hayato*; Sato, Tetsuya; Kikuchi, Takahiro; Nishinaka, Ichiro; et al.
Chemistry Letters, 38(11), p.1084 - 1085, 2009/10
Times Cited Count:17 Percentile:50.80(Chemistry, Multidisciplinary)We report on the characteristic anion-exchange behavior of the superheavy element dubnium (Db) with atomic number Z = 105 in HF/HNO solution at the fluoride ion concentration [F
solution at the fluoride ion concentration [F ] = 0.003 M. The result clearly demonstrates that the fluoro complex formation of Db is significantly different from that of the group-5 homologue Ta in the 6th period of the periodic table while the behavior of Db is similar to that of the lighter homologue Nb in the 5th period.
] = 0.003 M. The result clearly demonstrates that the fluoro complex formation of Db is significantly different from that of the group-5 homologue Ta in the 6th period of the periodic table while the behavior of Db is similar to that of the lighter homologue Nb in the 5th period.
Toyoshima, Atsushi; Kasamatsu, Yoshitaka*; Tsukada, Kazuaki; Asai, Masato; Kitatsuji, Yoshihiro; Ishii, Yasuo; Tome, Hayato; Nishinaka, Ichiro; Haba, Hiromitsu*; Oe, Kazuhiro*; et al.
Journal of the American Chemical Society, 131(26), p.9180 - 9181, 2009/07
Times Cited Count:16 Percentile:49.43(Chemistry, Multidisciplinary)We report here on the successful oxidation of element 102, nobelium (No), on an atom-at-a-time scale in 0.1 M  -hydroxyisobutyric acid (
-hydroxyisobutyric acid ( -HIB) solution using newly developed flow electrolytic column chromatography. It is found that the most stable No
-HIB) solution using newly developed flow electrolytic column chromatography. It is found that the most stable No is oxidized to No
is oxidized to No within 3 min and that the oxidized No complex with
within 3 min and that the oxidized No complex with  -HIB holds the trivalent state in the column above the applied potential of 1.0 V.
-HIB holds the trivalent state in the column above the applied potential of 1.0 V.
Suzuki, Chihiro*; Kato, Takako*; Sato, Kuninori*; Tamura, Naoki*; Kato, Daiji*; Sudo, Shigeru*; Yamamoto, Norimasa*; Tanuma, Hajime*; Ohashi, Hayato*; Suda, Shintaro*; et al.
Journal of Physics; Conference Series, 163, p.012019_1 - 012019_4, 2009/06
Times Cited Count:12 Percentile:93.87(Physics, Multidisciplinary)We have measured EUV spectra from highly charged tin ions in low density plasmas produced in the Large Helical Device (LHD). The well known dense spectral structure around 13.5 nm is measured when the plasma is rapidly cooled and approaching radioactive collapse, while the sparse spectrum with several unidentified discrete lines from 13.8-14.6 nm is observed if the plasma is cooled more slowly. The dominant charge states in the former case are Sn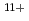 -Sn
-Sn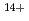 . The latter case may be explained by considering the spectral lines from charge states higher than Sn
. The latter case may be explained by considering the spectral lines from charge states higher than Sn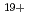 .
.
Haba, Hiromitsu*; Tsukada, Kazuaki; Asai, Masato; Toyoshima, Atsushi; Ishii, Yasuo; Tome, Hayato; Sato, Tetsuya; Nishinaka, Ichiro; Ichikawa, Takatoshi; Ichikawa, Shinichi; et al.
Radiochimica Acta, 95(1), p.1 - 6, 2007/01
Times Cited Count:16 Percentile:70.88(Chemistry, Inorganic & Nuclear)no abstracts in English
Hoshiba, Hideaki; Hashimoto, Makoto; Irokawa, H.; Usui, Toshihide; Sato, Hayato; Emori, Shuichi
JNC TN9410 2004-017, 170 Pages, 2004/08
While the JOYO MK-III Project, after the modification of primary coolant system started in oct.2000 and the integrated function tests, from Jun.2003, the performance test was executed for the purpose of verification of designed performance and confirmation of basic characteristics as an irradiation reactor. While the JOYO MK-III performance test, 28 tests were executed. Radiation control section took charge of 3 of them, "Dose Rate Distribution", "Radiation Control" and "Gaseous Waste Radioactive Concentration Measurement". The performance tests in charge of radiation control section was started on Jun.27, 2003, that is before the start-up of reactor, and were carried out when the thermal output of reactor was 40MWt, 70MWt, 105MWt and effective full power, 140MWt. The pre-operation tests in charge of radiation control section are "Test of dose rate measurement in operation and after shutdown". "Test of radioactive concentration measurement of air", and "Test of gaseous waste processing performance". The final test was "Test of dose rate measurement after shutdown", which was executed on Nov.27 2003. JOYO passed the inspection and the performance test was finished. The representative results in these performance tests are; 1.Every result is under the criterion 2.Dose rate and monitoring data are totally less than the data in MK-II operation. Though it confirmed that all the data are under the criterion, it is considered that these tests should be performed at proper intervals because the circumstances may change.
Onuki, Kaoru; Shimizu, Saburo; Nakajima, Hayato; Ikezoe, Yasumasa; Sato, Shoichi
International Journal of Hydrogen Energy, 15(2), p.93 - 97, 1990/00
Times Cited Count:0 Percentile:0.00(Chemistry, Physical)no abstracts in English
Nakajima, Hayato; Shimizu, Saburo; Onuki, Kaoru; Ikezoe, Yasumasa; Sato, Shoichi
Nihon Kagakkai-Shi, 1989(4), p.681 - 686, 1989/04
no abstracts in English
Sato, Shoichi; Shimizu, Saburo; Nakajima, Hayato; Ikezoe, Yasumasa
Int.J.Hydrogen Energy, 8(1), p.15 - 22, 1983/00
Times Cited Count:13 Percentile:85.30(Chemistry, Physical)no abstracts in English
Nagame, Yuichiro; Akiyama, Kazuhiko*; Asai, Masato; Goto, Shinichi*; Haba, Hiromitsu*; Ishii, Yasuo; Kasamatsu, Yoshitaka; Nishinaka, Ichiro; Sato, Tetsuya; Tome, Hayato*; et al.
no journal, ,
We present a study of fluoro complex formation of the transactinide elements, rutherfordium (Rf) and dubnium (Db), at JAEA (Japan Atomic Energy Agency). The transactinide nuclides  Rf and
Rf and  Db were produced in the reactions
Db were produced in the reactions  Cm(
Cm( O,5n) and
O,5n) and  Cm(
Cm( F,5n), respectively, at the JAEA tandem accelerator. Ion-exchange behavior of Rf and Db together with their lighter homologues in HF/HNO
F,5n), respectively, at the JAEA tandem accelerator. Ion-exchange behavior of Rf and Db together with their lighter homologues in HF/HNO mixed solutions has been investigated with a rapid ion-exchange separation apparatus AIDA. It has been found that fluoro complexation of Rf is significantly different from that of the homologues. A large difference in the adsorption behavior of Db and the homologue Ta on the anion-exchange resin has been also observed.
mixed solutions has been investigated with a rapid ion-exchange separation apparatus AIDA. It has been found that fluoro complexation of Rf is significantly different from that of the homologues. A large difference in the adsorption behavior of Db and the homologue Ta on the anion-exchange resin has been also observed.
Nagame, Yuichiro; Toyoshima, Atsushi; Ishii, Yasuo; Tsukada, Kazuaki; Asai, Masato; Tome, Hayato; Kasamatsu, Yoshitaka; Nishinaka, Ichiro; Sato, Tetsuya; Haba, Hiromitsu*; et al.
no journal, ,
We present a study of fluoride complex formation of Rf at Japan Atomic Energy Agency (JAEA). Anion- and cation-exchange behavior of Rf was systematically investigated in HF/HNO mixed solution together with the lighter homologues Zr and Hf using the automated ion-exchange separation apparatus coupled with the detection system for alpha spectroscopy. We unequivocally determined the species of Rf on the binding sites of the resin as the hexafluoro complex [RfF
mixed solution together with the lighter homologues Zr and Hf using the automated ion-exchange separation apparatus coupled with the detection system for alpha spectroscopy. We unequivocally determined the species of Rf on the binding sites of the resin as the hexafluoro complex [RfF ]
] . It is found that there is about two-orders of magnitude difference in the fluoride ion concentration between Rf and the homologues for the formation of the hexafluoro complexes. The result clearly demonstrates that the formation of the fluoride complexes of Rf is much weaker than those of the homologues Zr and Hf.
. It is found that there is about two-orders of magnitude difference in the fluoride ion concentration between Rf and the homologues for the formation of the hexafluoro complexes. The result clearly demonstrates that the formation of the fluoride complexes of Rf is much weaker than those of the homologues Zr and Hf.
 solutions
solutionsKasamatsu, Yoshitaka; Tome, Hayato; Toyoshima, Atsushi; Tsukada, Kazuaki; Asai, Masato; Ishii, Yasuo; Nishinaka, Ichiro; Sato, Tetsuya; Shinohara, Nobuo; Nagame, Yuichiro; et al.
no journal, ,
Anion-exchange behavior of  Db (half-life
Db (half-life 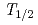 = 34 s) produced in the
= 34 s) produced in the  Cm(
Cm( F, 5
F, 5 ) reaction at the JAEA tandem accelerator was investigated in the mixed 0.89 M HF/0.3 M HNO
) reaction at the JAEA tandem accelerator was investigated in the mixed 0.89 M HF/0.3 M HNO solution ([F
solution ([F ] = 3
] = 3  10
10 M) with the automated ion-exchange separation apparatus coupled with the detection system for alpha-spectroscopy (AIDA). Anion-exchange behavior of its lighter homologues, Nb and Ta, was also studied under the same conditions using
M) with the automated ion-exchange separation apparatus coupled with the detection system for alpha-spectroscopy (AIDA). Anion-exchange behavior of its lighter homologues, Nb and Ta, was also studied under the same conditions using  Nb (
Nb (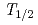 = 14.3 min) and
= 14.3 min) and  Ta (
Ta (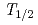 = 6.76 min) produced in the
= 6.76 min) produced in the 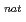 Ge(
Ge( F,
F, 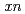 ) and
) and 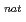 Gd(
Gd( F,
F, 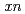 ) reactions, respectively. It was found that the adsorption probability on the anion-exchange resin is in the order of Ta
) reactions, respectively. It was found that the adsorption probability on the anion-exchange resin is in the order of Ta  Nb
Nb  Db under the present condition.
Db under the present condition.
Tsukada, Kazuaki; Toyoshima, Atsushi; Haba, Hiromitsu*; Asai, Masato; Akiyama, Kazuhiko*; Ishii, Yasuo; Tome, Hayato*; Nishinaka, Ichiro; Sato, Tetsuya; Ichikawa, Takatoshi; et al.
no journal, ,
no abstracts in English
 media
mediaKasamatsu, Yoshitaka; Toyoshima, Atsushi; Asai, Masato; Tsukada, Kazuaki; Haba, Hiromitsu*; Ishii, Yasuo; Tome, Hayato; Nishinaka, Ichiro; Akiyama, Kazuhiko*; Kikunaga, Hidetoshi*; et al.
no journal, ,
Dubnium-262 was produced in the  Cm(
Cm( F, 5
F, 5 ) reaction at the JAEA tandem accelerator. Adsorption of Db on the anion-exchange resin was investigated in 0.89 M HF/0.3 M HNO
) reaction at the JAEA tandem accelerator. Adsorption of Db on the anion-exchange resin was investigated in 0.89 M HF/0.3 M HNO solution. The anion-exchange behavior of Nb, Ta, and Pa as homologues of Db was also examined in details in HF/HNO
solution. The anion-exchange behavior of Nb, Ta, and Pa as homologues of Db was also examined in details in HF/HNO solutions. From the comparison of those results, we found that the adsorption of Db on the anion-exchange resin is considerably weaker than that of Ta and is relatively similar to those of Nb and Pa in the studied conditions.
solutions. From the comparison of those results, we found that the adsorption of Db on the anion-exchange resin is considerably weaker than that of Ta and is relatively similar to those of Nb and Pa in the studied conditions.