Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Nagai, Takayuki; Okamoto, Yoshihiro; Shibata, Daisuke*; Kojima, Kazuo*; Hasegawa, Takehiko*; Sato, Seiichi*; Fukaya, Akane*; Hatakeyama, Kiyoshi*
JAEA-Research 2024-014, 54 Pages, 2025/02
XAFS measurements in the soft X-ray region are suitable for evaluating the chemical state of the surface layer of a measurement sample because the X-ray transmittance is low. In this study, the purpose of the study was to confirm the difference between the coagulated surface layer and the inside of the simulated waste glasses by measuring the K-edge of the glass constituent elements boron, oxygen, sodium, and silicon, and the L edge of the waste component cerium. As a result, the B K-edge XANES spectra showed that the proportion of B-O tetracoordinate sp
edge of the waste component cerium. As a result, the B K-edge XANES spectra showed that the proportion of B-O tetracoordinate sp structures (BO
structures (BO ) on the surface layer of the coagulated glass samples was higher than that on the cut surface inside the glass samples, which is expected to improve the water resistance of the coagulated surface. On the other hand, the O K-edge XANES spectra suggested that the O abundance in the coagulated surface layer was lower than that in the cut surface inside the glass samples, and that alkali metal elements may be concentrated in the coagulated surface layer. However, no difference was observed in the Na K-edge XANES spectra between the coagulated surface layer and the cut surface, and no difference was observed in the Si K-edge XANES spectra between the solidified surface and the inside of glass samples. In addition, the Ce L
) on the surface layer of the coagulated glass samples was higher than that on the cut surface inside the glass samples, which is expected to improve the water resistance of the coagulated surface. On the other hand, the O K-edge XANES spectra suggested that the O abundance in the coagulated surface layer was lower than that in the cut surface inside the glass samples, and that alkali metal elements may be concentrated in the coagulated surface layer. However, no difference was observed in the Na K-edge XANES spectra between the coagulated surface layer and the cut surface, and no difference was observed in the Si K-edge XANES spectra between the solidified surface and the inside of glass samples. In addition, the Ce L -edge XANES spectra confirmed that the Ce valence in the surface layer of coagulated glass samples were oxidized compared to the inside of glass samples.
-edge XANES spectra confirmed that the Ce valence in the surface layer of coagulated glass samples were oxidized compared to the inside of glass samples.
Sato, Yuki; Kakuto, Takeshi*; Tanaka, Takayuki*; Shimano, Hiroyuki*
European Physical Journal; Special Topics, 10 Pages, 2025/00
Times Cited Count:0 Percentile:0.00(Physics, Multidisciplinary)Kobayashi, Taishi*; Sato, Yutaro*; Tonna, Ryutaro*; Matsumura, Daiju; Sasaki, Takayuki*; Ikeda, Atsushi
Dalton Transactions (Internet), 53(46), p.18616 - 18628, 2024/10
Times Cited Count:0 Percentile:0.00(Chemistry, Inorganic & Nuclear)Sato, Nobuaki*; Kameo, Yutaka; Sato, Soichi; Kumagai, Yuta; Sato, Tomonori; Yamamoto, Masahiro*; Watanabe, Yutaka*; Nagai, Takayuki; Niibori, Yuichi*; Watanabe, Masayuki; et al.
Introduction to Dismantling and Decommissioning Chemistry, 251 Pages, 2024/09
This book focuses on the dismantling and decommissioning of nuclear facilities and reactors that have suffered severe accidents. In Part 1, we introduce basic aspects ranging from fuel chemistry, analytical chemistry, radiation chemistry, corrosion, and decontamination chemistry to waste treatment and disposal. Then, Part 2 covers the chemistry involved in the decommissioning of various nuclear facilities, and discusses what chemical approaches are necessary and possible for the decommissioning of TEPCO's Fukushima Daiichi Nuclear Power Plants, how decommissioning should be carried out, and what kind of research and development and also human resource development are required for this.
Katsuoka, Nanako; Akiyama, Daisuke*; Kirishima, Akira*; Nagai, Takayuki; Okamoto, Yoshihiro; Sato, Nobuaki*
2023-Nendo "Busshitsu, Debaisu Ryoiki Kyodo Kenkyu Kyoten" Kenkyu Seika, Katsudo Hokokusho (Internet), 1 Pages, 2024/07
no abstracts in English
Sato, Yuki; Kakuto, Takeshi*; Tanaka, Takayuki*; Shimano, Hiroyuki*; Morohashi, Yuko; Hatakeyama, Tomoyoshi*; Nakajima, Junsaku; Ishiyama, Masahiro
Nuclear Instruments and Methods in Physics Research A, 1063, p.169300_1 - 169300_7, 2024/06
Times Cited Count:3 Percentile:86.15(Instruments & Instrumentation)Tonna, Ryutaro*; Sasaki, Takayuki*; Okamoto, Yoshihiro; Kobayashi, Taishi*; Akiyama, Daisuke*; Kirishima, Akira*; Sato, Nobuaki*
Journal of Nuclear Materials, 589, p.154862_1 - 154862_10, 2024/02
Times Cited Count:2 Percentile:43.92(Materials Science, Multidisciplinary)The dissolution behavior of FeUO compounds formed by a high-temperature reaction of UO
compounds formed by a high-temperature reaction of UO with iron, a stainless-steel component of reactor structural materials, was investigated under atmospheric conditions. The compounds were prepared in an electric furnace using U
with iron, a stainless-steel component of reactor structural materials, was investigated under atmospheric conditions. The compounds were prepared in an electric furnace using U O
O and Fe
and Fe O
O as starting materials, and their solid states were analyzed using X-ray diffraction, scanning electron microscopy energy dispersive X-ray spectroscopy, and X-ray absorption fine structure spectroscopy. The concentration of nuclides dissolved in water was examined by performing static leaching tests of FeUO
as starting materials, and their solid states were analyzed using X-ray diffraction, scanning electron microscopy energy dispersive X-ray spectroscopy, and X-ray absorption fine structure spectroscopy. The concentration of nuclides dissolved in water was examined by performing static leaching tests of FeUO compounds for up to three months. A redox reaction was proposed to occur between trivalent Fe and pentavalent U ions in the early stage of FeUO
compounds for up to three months. A redox reaction was proposed to occur between trivalent Fe and pentavalent U ions in the early stage of FeUO dissolution. It was thermodynamically deduced that the reduced divalent Fe ion was finally oxidized into a trivalent ion in the presence of dissolved oxygen, and iron hydroxide limited the solubility of Fe. Meanwhile, the concentration of hexavalent U (i.e., uranyl ion) was limited owing to the presence of secondary minerals such as metaschoepite and sodium uranate and subsequently decreased, possibly owing to sorption on Fe oxides, for example. The concentrations of multivalent ions of fission products, such as Ru and Ce, also decreased, likely for the reason above. By contrast, the concentration of soluble Cs ions did not decrease. The validity of this interpretation was supported by comparing the results with the dissolution behavior of a reference sample (Fe-free U
dissolution. It was thermodynamically deduced that the reduced divalent Fe ion was finally oxidized into a trivalent ion in the presence of dissolved oxygen, and iron hydroxide limited the solubility of Fe. Meanwhile, the concentration of hexavalent U (i.e., uranyl ion) was limited owing to the presence of secondary minerals such as metaschoepite and sodium uranate and subsequently decreased, possibly owing to sorption on Fe oxides, for example. The concentrations of multivalent ions of fission products, such as Ru and Ce, also decreased, likely for the reason above. By contrast, the concentration of soluble Cs ions did not decrease. The validity of this interpretation was supported by comparing the results with the dissolution behavior of a reference sample (Fe-free U O
O ).
).
Sato, Nobuaki*; Kirishima, Akira*; Sasaki, Takayuki*; Takano, Masahide; Kumagai, Yuta; Sato, Soichi; Tanaka, Kosuke
Current Location of Fuel Debris Chemistry, 178 Pages, 2023/11
Considerable efforts have been devoted to the decommissioning of the TEPCO's Fukushima Daiichi Nuclear Power Station (1F) and now the retrieval of fuel debris is being proceeded on a trial basis. It can be said that the succession of science and technology related to debris, that is, human resource development, is important and indispensable. For that reason, we thought that a specific textbook on decommissioning is necessary. Regarding the 1F fuel debris, we still do not know enough, and it would be difficult to describe the details. However, 12 years have passed since the accident, and we have come to understand the situation of 1F to a certain extent. At this stage, it is essential for future development to organize the current situation by combining examples of past severe accidents. Therefore, we presented in this book the current state of fuel debris chemistry research from the perspectives of solid chemistry, solution chemistry, analytical chemistry, radiochemistry, and radiation chemistry.
Nagai, Takayuki; Okamoto, Yoshihiro; Yamagishi, Hirona*; Shibata, Daisuke*; Kojima, Kazuo*; Hasegawa, Takehiko*; Sato, Seiichi*; Fukaya, Akane*; Hatakeyama, Kiyoshi*
JAEA-Research 2023-004, 45 Pages, 2023/09
The local structure of glass-forming elements and waste elements in borosilicate glasses varies with its chemical composition. In this study, simulated waste glass samples were prepared and the chemical state regarding boron (B), silicon (Si) and waste elements of iron (Fe), cesium (Cs) were estimated by using XAFS measurement in soft X-ray region. To understand the chemical stability of simulated waste glasses, XANES spectra of B K-edge, Fe L , L
, L -edge, and Cs M
-edge, and Cs M , M
, M -edge were measured on the glass surface exposed to the leachate. As a result, it was found that the glass surface exposed to the leachate was changed and it was difficult to obtain a clear XANES spectrum. From the B K-edge XANES spectra on glass surfaces exposed to the leachate, an increase in three-coordination of B-O (BO
-edge were measured on the glass surface exposed to the leachate. As a result, it was found that the glass surface exposed to the leachate was changed and it was difficult to obtain a clear XANES spectrum. From the B K-edge XANES spectra on glass surfaces exposed to the leachate, an increase in three-coordination of B-O (BO ) and a decrease in four-coordination of B-O (BO
) and a decrease in four-coordination of B-O (BO ) were observed compared to the glass surfaces before immersion. The XANES spectra of Fe L
) were observed compared to the glass surfaces before immersion. The XANES spectra of Fe L , L
, L -edge, and Cs M
-edge, and Cs M , M
, M -edge show that as the exposure time in the leachate increases, the Cs present on the glass surface dissolves into the leachate. The XANES spectra of Si K-edge were measured on simulated waste glass surfaces before immersion, and it was confirmed that the change in XANES spectra given by Na
-edge show that as the exposure time in the leachate increases, the Cs present on the glass surface dissolves into the leachate. The XANES spectra of Si K-edge were measured on simulated waste glass surfaces before immersion, and it was confirmed that the change in XANES spectra given by Na O concentration had a larger effect than the waste component concentration.
O concentration had a larger effect than the waste component concentration.
Nagai, Takayuki; Akiyama, Daisuke*; Kirishima, Akira*; Katsuoka, Nanako; Okamoto, Yoshihiro; Sato, Nobuaki*
2022-Nendo "Busshitsu, Debaisu Ryoiki Kyodo Kenkyu Kyoten" Kenkyu Seika, Katsudo Hokokusho (Internet), 1 Pages, 2023/09
no abstracts in English
Kusaka, Ryoji; Kumagai, Yuta; Watanabe, Masayuki; Sasaki, Takayuki*; Akiyama, Daisuke*; Sato, Nobuaki*; Kirishima, Akira*
Journal of Nuclear Science and Technology, 60(5), p.603 - 613, 2023/05
Times Cited Count:6 Percentile:60.29(Nuclear Science & Technology) , Zr, and stainless steel and leaching behavior of the fission products and matrix elements
, Zr, and stainless steel and leaching behavior of the fission products and matrix elementsTonna, Ryutaro*; Sasaki, Takayuki*; Kodama, Yuji*; Kobayashi, Taishi*; Akiyama, Daisuke*; Kirishima, Akira*; Sato, Nobuaki*; Kumagai, Yuta; Kusaka, Ryoji; Watanabe, Masayuki
Nuclear Engineering and Technology, 55(4), p.1300 - 1309, 2023/04
Times Cited Count:6 Percentile:84.29(Nuclear Science & Technology)Simulated debris was synthesized using UO , Zr, and stainless steel and a heat treatment method under inert or oxidizing conditions. The primary U solid phase of the debris synthesized at 1473 K under inert conditions was UO
, Zr, and stainless steel and a heat treatment method under inert or oxidizing conditions. The primary U solid phase of the debris synthesized at 1473 K under inert conditions was UO , whereas a (U,Zr)O
, whereas a (U,Zr)O solid solution formed at 1873 K. Under oxidizing conditions, a mixture of U
solid solution formed at 1873 K. Under oxidizing conditions, a mixture of U O
O and (Fe,Cr)UO
and (Fe,Cr)UO phases formed at 1473 K whereas a (U,Zr)O
phases formed at 1473 K whereas a (U,Zr)O solid solution formed at 1873 K. The leaching behavior of the fission products from the simulated debris was evaluated using two methods: the irradiation method, for which fission products were produced via neutron irradiation, and the doping method, for which trace amounts of non-radioactive elements were doped into the debris. The dissolution behavior of U depended on the properties of the debris and aqueous medium the debris was immersed in. Cs, Sr, and Ba leached out regardless of the primary solid phases. The leaching of high-valence Eu and Ru ions was suppressed, possibly owing to their solid-solution reaction with or incorporation into the uranium compounds of the simulated debris.
solid solution formed at 1873 K. The leaching behavior of the fission products from the simulated debris was evaluated using two methods: the irradiation method, for which fission products were produced via neutron irradiation, and the doping method, for which trace amounts of non-radioactive elements were doped into the debris. The dissolution behavior of U depended on the properties of the debris and aqueous medium the debris was immersed in. Cs, Sr, and Ba leached out regardless of the primary solid phases. The leaching of high-valence Eu and Ru ions was suppressed, possibly owing to their solid-solution reaction with or incorporation into the uranium compounds of the simulated debris.
Okamoto, Yoshihiro; Shiwaku, Hideaki; Shimamura, Keisuke*; Kobayashi, Hidekazu; Nagai, Takayuki; Inose, Takehiko*; Sato, Seiichi*; Hatakeyama, Kiyoshi*
Journal of Nuclear Materials, 570, p.153962_1 - 153962_13, 2022/11
Simulated nuclear waste glass samples containing phosphorus, which increase the solubility of molybdenum, were prepared and analyzed using synchrotron X-ray Absorption Fine Structure (XAFS) analysis for some constituent elements and Raman spectroscopic analysis of their complex structure. Changes in local structure and chemical state due to different phosphorus additions and waste loading rates were systematically studied. Consequently, no crystalline phase due to the molybdate compound was observed even at a maximum waste content of 30 wt% (corresponding to 1.87 mol% MoO ). Oxidation proceeded when the waste-loading rate was increased, whereas the reduction proceeded when phosphorus was added. In some cases, the effects of oxidation and reduction were offset. The local structure around specific elements can be classified as follows; Zn that is affected mainly by the waste-loading rate, Ce that is affected by both the waste-loading rate and phosphorus addition, and Zr element that is not affected by either of them. From the comparison between the analytical results of Mo and other elements, it was considered that the added phosphorus exists as a free PO
). Oxidation proceeded when the waste-loading rate was increased, whereas the reduction proceeded when phosphorus was added. In some cases, the effects of oxidation and reduction were offset. The local structure around specific elements can be classified as follows; Zn that is affected mainly by the waste-loading rate, Ce that is affected by both the waste-loading rate and phosphorus addition, and Zr element that is not affected by either of them. From the comparison between the analytical results of Mo and other elements, it was considered that the added phosphorus exists as a free PO structural unit and may deprive the alkali metal coordinated to the molybdate ion.
structural unit and may deprive the alkali metal coordinated to the molybdate ion.
Nagai, Takayuki; Okamoto, Yoshihiro; Yamagishi, Hirona*; Kojima, Kazuo*; Inose, Takehiko*; Sato, Seiichi*; Hatakeyama, Kiyoshi*
JAEA-Research 2022-008, 37 Pages, 2022/10
The local structure of glass-forming elements and waste elements in borosilicate glasses varies with its chemical composition. In this study, borosilicate glass frit and simulated waste glass samples were prepared and the chemical state regarding boron (B), silicon (Si) and waste elements of iron (Fe), cesium (Cs) were estimated by using XAFS measurement in soft X-ray region. Following results were obtained by XAFS measurements of simulated waste glass surfaces after immersion test to investigate the long chemical stability. (1) As the leaching time of glass samples in immersion test passed, the Cs M , M
, M -edge XANES spectra disappeared and the Fe L
-edge XANES spectra disappeared and the Fe L , L
, L -edge spectra changed. (2) A new compound was formed on the sample surface after the immersion test, and these changes in the surface state were confirmed by Raman spectroscopy. However, it became difficult to obtain a clear B K-edge XANES spectrum by forming a compound on glass surfaces. The Si K-edge XANES spectra of borosilicate glass frits with different Na
-edge spectra changed. (2) A new compound was formed on the sample surface after the immersion test, and these changes in the surface state were confirmed by Raman spectroscopy. However, it became difficult to obtain a clear B K-edge XANES spectrum by forming a compound on glass surfaces. The Si K-edge XANES spectra of borosilicate glass frits with different Na O content were measured, and following was confirmed. (1) As the Na
O content were measured, and following was confirmed. (1) As the Na O concentration increases in borosilicate glass frit, the K-edge peak of Si shifts to the low energy side. (2) The intensity of the Si K-edge peak is maximum when the Na
O concentration increases in borosilicate glass frit, the K-edge peak of Si shifts to the low energy side. (2) The intensity of the Si K-edge peak is maximum when the Na O content in glass frits was about 7wt%.
O content in glass frits was about 7wt%.
Takeuchi, Yusuke*; Tojo, Junji*; Yamanaka, T.*; Nakazawa, Yuga*; Iinuma, Hiromi*; Kondo, Yasuhiro; Kitamura, Ryo; Morishita, Takatoshi; Cicek, E.*; Ego, Hiroyasu*; et al.
Proceedings of 31st International Linear Accelerator Conference (LINAC 2022) (Internet), p.562 - 564, 2022/10
A muon linac is under development for future muon g-2/EDM experiments at J-PARC. The linac provides a 212 MeV muon beam to an MRI-type compact storage ring. After the initial acceleration using the electrostatic field created by mesh and cylindrical electrodes, the muons are accelerated using four types of radio-frequency accelerators. To validate the linac design as a whole, end-to-end simulations were performed using General Particle Tracer. In addition, error studies were performed to investigate the effects on beam and spin dynamics of various errors in the accelerator components and input beam distribution. This paper describes the results of the end-to-end simulations and error studies.
 and CrUO
and CrUO
Akiyama, Daisuke*; Kusaka, Ryoji; Kumagai, Yuta; Nakada, Masami; Watanabe, Masayuki; Okamoto, Yoshihiro; Nagai, Takayuki; Sato, Nobuaki*; Kirishima, Akira*
Journal of Nuclear Materials, 568, p.153847_1 - 153847_10, 2022/09
Times Cited Count:4 Percentile:51.78(Materials Science, Multidisciplinary)FeUO , CrUO
, CrUO , and Fe
, and Fe Cr
Cr UO
UO are monouranates containing pentavalent U. Even though these compounds have similar crystal structures, their formation conditions and thermal stability are significantly different. To determine the factors causing the difference in thermal stability between FeUO
are monouranates containing pentavalent U. Even though these compounds have similar crystal structures, their formation conditions and thermal stability are significantly different. To determine the factors causing the difference in thermal stability between FeUO and CrUO
and CrUO , their crystal structures were evaluated in detail. A Raman band was observed at 700 cm
, their crystal structures were evaluated in detail. A Raman band was observed at 700 cm in all the samples. This Raman band was derived from the stretching vibration of the O-U-O axis band, indicating that Fe
in all the samples. This Raman band was derived from the stretching vibration of the O-U-O axis band, indicating that Fe Cr
Cr UO
UO was composed of a uranyl-like structure in its lattice regardless of its "x"' value. M
was composed of a uranyl-like structure in its lattice regardless of its "x"' value. M ssbauer measurements indicated that the Fe in FeUO
ssbauer measurements indicated that the Fe in FeUO and Fe
and Fe Cr
Cr UO
UO were trivalent. Furthermore, Fe
were trivalent. Furthermore, Fe Cr
Cr UO
UO lost its symmetry around Fe
lost its symmetry around Fe with increasing electron densities around Fe
with increasing electron densities around Fe , as the abundance of Cr increased. These results suggested no significant structural differences between FeUO
, as the abundance of Cr increased. These results suggested no significant structural differences between FeUO and CrUO
and CrUO . Thermogravimetric measurements for UO
. Thermogravimetric measurements for UO , FeUO
, FeUO , and CrUO
, and CrUO showed that the temperature at which FeUO
showed that the temperature at which FeUO decomposed under an oxidizing condition (approximately 800
decomposed under an oxidizing condition (approximately 800  C) was significantly lower than the temperature at which the decomposition of CrUO
C) was significantly lower than the temperature at which the decomposition of CrUO started (approximately 1250
started (approximately 1250  C). Based on these results, we concluded that the decomposition of FeUO
C). Based on these results, we concluded that the decomposition of FeUO was triggered by an "in-crystal" redox reaction, i.e., Fe
was triggered by an "in-crystal" redox reaction, i.e., Fe
 U
U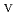
 Fe
Fe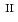
 U
U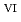 , which would not occur in the CrUO
, which would not occur in the CrUO lattice because Cr
lattice because Cr could never be reduced under the investigated condition. Finally, the existence of Cr
could never be reduced under the investigated condition. Finally, the existence of Cr in FexCr
in FexCr UO
UO effectively suppressed the decomposition of the Fe
effectively suppressed the decomposition of the Fe Cr
Cr UO
UO crystal, even at a very low Cr content.
crystal, even at a very low Cr content.
 , Zr, and stainless-steel
, Zr, and stainless-steelKirishima, Akira*; Akiyama, Daisuke*; Kumagai, Yuta; Kusaka, Ryoji; Nakada, Masami; Watanabe, Masayuki; Sasaki, Takayuki*; Sato, Nobuaki*
Journal of Nuclear Materials, 567, p.153842_1 - 153842_15, 2022/08
Times Cited Count:9 Percentile:81.25(Materials Science, Multidisciplinary)To understand the chemical structure and stability of nuclear fuel debris consisting of UO , Zr, and Stainless Steel (SUS) generated by the Fukushima Daiichi Nuclear Power Plant accident in Japan in 2011, simulated debris of the UO
, Zr, and Stainless Steel (SUS) generated by the Fukushima Daiichi Nuclear Power Plant accident in Japan in 2011, simulated debris of the UO -SUS-Zr system and other fundamental component systems were synthesized and characterized. The simulated debris were synthesized by heat treatment for 1 to 12 h at 1600
-SUS-Zr system and other fundamental component systems were synthesized and characterized. The simulated debris were synthesized by heat treatment for 1 to 12 h at 1600 C, in inert (Ar) or oxidative (Ar + 2% O
C, in inert (Ar) or oxidative (Ar + 2% O ) atmospheres.
) atmospheres.  Np and
Np and  Am tracers were doped for the leaching tests of these elements and U from the simulated debris. The characterization of the simulated debris was conducted by XRD, SEM-EDX, Raman spectroscopy, and M
Am tracers were doped for the leaching tests of these elements and U from the simulated debris. The characterization of the simulated debris was conducted by XRD, SEM-EDX, Raman spectroscopy, and M ssbauer spectroscopy, which provided the major uranium phase of the UO
ssbauer spectroscopy, which provided the major uranium phase of the UO  -SUS-Zr debris was the solid solution of U
-SUS-Zr debris was the solid solution of U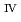 O
O (s.s.) with Zr(IV) and Fe(II) regardless of the treatment atmosphere. The long-term immersion test of the simulated debris in pure water and that in seawater revealed the macro scale crystal structure of the simulated debris was chemically very stable in the wet condition for a year or more. Furthermore, the leaching test results showed that the actinide leaching ratios of U, Np, Am from the UO
(s.s.) with Zr(IV) and Fe(II) regardless of the treatment atmosphere. The long-term immersion test of the simulated debris in pure water and that in seawater revealed the macro scale crystal structure of the simulated debris was chemically very stable in the wet condition for a year or more. Furthermore, the leaching test results showed that the actinide leaching ratios of U, Np, Am from the UO -SUS-Zr debris were very limited and less than 0.08 % for all the experiments in this study.
-SUS-Zr debris were very limited and less than 0.08 % for all the experiments in this study.
 O
O solution
solutionKumagai, Yuta; Kusaka, Ryoji; Nakada, Masami; Watanabe, Masayuki; Akiyama, Daisuke*; Kirishima, Akira*; Sato, Nobuaki*; Sasaki, Takayuki*
Journal of Nuclear Science and Technology, 59(8), p.961 - 971, 2022/08
Times Cited Count:3 Percentile:41.50(Nuclear Science & Technology)We investigated potential degradation of fuel debris caused by H O
O , which is the oxidant of major impact from water radiolysis. We performed leaching experiments on different kinds of simulated debris comprising U, Fe, Cr, Ni, and Zr in an aqueous H
, which is the oxidant of major impact from water radiolysis. We performed leaching experiments on different kinds of simulated debris comprising U, Fe, Cr, Ni, and Zr in an aqueous H O
O solution. Chemical analysis of the leaching solution showed that U dissolution was induced by H
solution. Chemical analysis of the leaching solution showed that U dissolution was induced by H O
O . Raman analysis after the leaching revealed that uranyl peroxides were formed on the surface of the simulated debris. These results demonstrate that uranyl peroxides are possible alteration products of fuel debris from H
. Raman analysis after the leaching revealed that uranyl peroxides were formed on the surface of the simulated debris. These results demonstrate that uranyl peroxides are possible alteration products of fuel debris from H O
O reaction. However, the sample in which the main uranium-containing phase was a U-Zr oxide solid solution showed much less uranium dissolution and no Raman signal of uranyl peroxides. Comparison of these results indicates that formation of an oxide solid solution of Zr with UO
reaction. However, the sample in which the main uranium-containing phase was a U-Zr oxide solid solution showed much less uranium dissolution and no Raman signal of uranyl peroxides. Comparison of these results indicates that formation of an oxide solid solution of Zr with UO improves the stability of fuel debris against H
improves the stability of fuel debris against H O
O reaction.
reaction.
Senzaki, Tatsuya; Arai, Yoichi; Yano, Kimihiko; Sato, Daisuke; Tada, Kohei; Ogi, Hiromichi*; Kawanobe, Takayuki*; Ono, Shimpei; Nakamura, Masahiro; Kitawaki, Shinichi; et al.
JAEA-Testing 2022-001, 28 Pages, 2022/05
In preparation for the decommissioning of Laboratory B of the Nuclear Fuel Cycle Engineering Laboratory, the nuclear fuel material that had been stored in the glove box for a long time was moved to the Chemical Processing Facility (CPF). This nuclear fuel material was stored with sealed by a polyvinyl chloride (PVC) bag in the storage. Since it was confirmed that the PVC bag swelled during storage, it seems that any gas was generated by radiolysis of the some components contained in the nuclear fuel material. In order to avoid breakage of the PVC bag and keep it safety for long time, we began the study on the stabilization treatment of the nuclear fuel material. First, in order to clarify the properties of nuclear fuel material, radioactivity analysis, component analysis, and thermal analysis were carried out. From the results of thermal analysis, the existence of organic matter was clarified. Then, ion exchange resin with similar thermal characteristics was selected and the thermal decomposition conditions were investigated. From the results of these analyzes and examinations, the conditions for thermal decomposition of the nuclear fuel material contained with organic matter was established. Performing a heat treatment of a small amount of nuclear fuel material in order to confirm the safety, after which the treatment amount was scaled up. It was confirmed by the weight change after the heat treatment that the nuclear fuel material contained with organic matter was completely decomposed.
 O
O -induced oxidative degradation of simulated fuel debris
-induced oxidative degradation of simulated fuel debrisKumagai, Yuta; Kusaka, Ryoji; Nakada, Masami; Watanabe, Masayuki; Akiyama, Daisuke*; Kirishima, Akira*; Sato, Nobuaki*; Sasaki, Takayuki*
Hoshasen Kagaku (Internet), (113), p.61 - 64, 2022/04
The severe accident at TEPCO's Fukushima Daiichi Nuclear Power Station resulted in generation of fuel debris. The fuel debris is in contact with water and the radiolysis of water can accelerate degradation of the debris. The analysis of particles sampled from inside or near the damaged reactors indicates the complicated compositions of the fuel debris. It is challenging to estimate the effect of water radiolysis on such a complicated material. Therefore, in this study, we investigated the potential degradation process by leaching experiments of simulated fuel debris in aqueous H O
O solution. The results show that the reaction of H
solution. The results show that the reaction of H O
O induced uranium dissolution from most of the samples and then formation of uranyl peroxides. In contrast, a sample that had U-Zr oxide solid solution as the major phase exhibited remarkable resistance to H
induced uranium dissolution from most of the samples and then formation of uranyl peroxides. In contrast, a sample that had U-Zr oxide solid solution as the major phase exhibited remarkable resistance to H O
O . These findings revealed that the degradation of the simulated debris reflects the reactivity and stability of the uranium phase in the matrices.
. These findings revealed that the degradation of the simulated debris reflects the reactivity and stability of the uranium phase in the matrices.