Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Savage, D.*; Wilson, J.*; Benbow, S.*; Sasamoto, Hiroshi; Oda, Chie; Walker, C.*; Kawama, Daisuke*; Tachi, Yukio
Applied Clay Science, 195, p.105741_1 - 105741_11, 2020/09
Times Cited Count:4 Percentile:11.39(Chemistry, Physical)Safety functions for the clay buffer in a repository for high-level radioactive waste (HLW) are fulfilled if the presence of montmorillonite with high swelling capacity and low permeability is maintained in the long-term. The transformation of montmorillonite to the non-swelling mineral likely illite is addressed in most safety assessments by using simple semi-empirical kinetic models, but this approach contrasts with more complex reactive-transport simulations. In the present study, reactive-transport simulations are compared with simple semi-empirical kinetic models. Results suggest that reactive-transport simulations err on the side of conservatism, but may produce unrealistic estimates of illitization. This comparison demonstrates that reactive-transport models may be carefully applied to simulate the long-term evolution of near field environment for HLW disposal.
Savage, D.*; Wilson, J.*; Benbow, S.*; Sasamoto, Hiroshi; Oda, Chie; Walker, C.*; Kawama, Daisuke*; Tachi, Yukio
Applied Clay Science, 179, p.105146_1 - 105146_10, 2019/10
Times Cited Count:13 Percentile:52.14(Chemistry, Physical)Natural systems evidence for the effects of temperature and the activity of aqueous silica upon montmorillonite stability was evaluated. Thermodynamic modeling using three different TDBs shows that stability fields for montmorillonite exist from 0 to 140 C, but at low values of silica activity, a stability field for illite replaces that for montmorillonite. Pore fluid chemical and mineralogical data for sediments from ODP sites from offshore Japan show a trend from montmorillonite + amorphous silica stability at temperatures up to 60
C, but at low values of silica activity, a stability field for illite replaces that for montmorillonite. Pore fluid chemical and mineralogical data for sediments from ODP sites from offshore Japan show a trend from montmorillonite + amorphous silica stability at temperatures up to 60 C to that for illite + quartz at higher temperatures. However, even over very long timescales (
C to that for illite + quartz at higher temperatures. However, even over very long timescales ( 1 Ma), smectite does not transform to illite under thermodynamically-favourable conditions at temperatures less than 80
1 Ma), smectite does not transform to illite under thermodynamically-favourable conditions at temperatures less than 80 C.
C.
Savage, D.*; Soler, J. M.*; Yamaguchi, Kohei; Walker, C.; Honda, Akira; Inagaki, Manabu; Watson, C.*; Wilson, J.*; Benbow, S.*; Gaus, I.*; et al.
Applied Geochemistry, 26(7), p.1138 - 1152, 2011/07
Times Cited Count:20 Percentile:47.74(Geochemistry & Geophysics)The use of cement and concrete as fracture grouting or as tunnel seals in a geological disposal facility for rad wastes creates potential issues concerning chemical reactivity. From a long term safety perspective, it is desirable to be able model these interactions and changes quantitatively. As part of the LCS (Long-term Cement Studies) project programme, a modelling inter-comparison has been conducted, involving the modelling of two experiments describing cement hadration and cement-rock reaction, with teams representing the NDA (UK), Posiva (Finland), and JAEA. This modelling exercise showed that the dominant reaction pathways in the two experiments are fairly well understood and are consistent between the different modelling teams, although significant differences existed amongst the precise parameterisation. Future modelling exercises of this type should focus on a suitable natural or industrial analogue that might aid assessing mineral-fluid reactions at these longer timescales.
Savage, D.*; Benbow, S.*; Watson, C.*; Takase, Hiroyasu*; Ono, Kaori*; Oda, Chie; Honda, Akira
Applied Clay Science, 47(1-2), p.72 - 81, 2010/01
Times Cited Count:38 Percentile:69.92(Chemistry, Physical)Mudstones containing smectite have been altered under mildly alkaline conditions (9  pH
pH  10) at Searles Lake, California over a 3 million-year time period. This natural alteration has been simulated incorporating time-dependent boundary conditions of sedimentation and fluid composition, a Pitzer model for activities of aqueous species, and a coupled hydrogeological model for time-dependent flow in the sediment layers. Kinetic dissolution of detrital smectite under alkaline conditions was described using one of two models based on departure from thermodynamic equilibrium or by an empirical rate dependent upon aqueous Si concentrations. The zonal pattern of smectite dissolution observed at Searles Lake was reproduced reasonably well by the "Cama-TST" model of montmorillonite dissolution. This assessment provides a test of the accuracy and reliability of published data in the application of models of smectite dissolution in the long-term.
10) at Searles Lake, California over a 3 million-year time period. This natural alteration has been simulated incorporating time-dependent boundary conditions of sedimentation and fluid composition, a Pitzer model for activities of aqueous species, and a coupled hydrogeological model for time-dependent flow in the sediment layers. Kinetic dissolution of detrital smectite under alkaline conditions was described using one of two models based on departure from thermodynamic equilibrium or by an empirical rate dependent upon aqueous Si concentrations. The zonal pattern of smectite dissolution observed at Searles Lake was reproduced reasonably well by the "Cama-TST" model of montmorillonite dissolution. This assessment provides a test of the accuracy and reliability of published data in the application of models of smectite dissolution in the long-term.
Wilson, J.*; Savage, D.*; Cuadros, J.*; Shibata, Masahiro; Ragnarsdottir, K. V.*
Geochimica et Cosmochimica Acta, 70(2), p.306 - 322, 2006/01
Times Cited Count:95 Percentile:57.55(Geochemistry & Geophysics)In some geological repository designs, steel canister and bentonite backfill are placed in contact. Some previous studies indicate that the montmorillonite component of the backfill could react with steel corrosion products to produce non-swelling Fe-rich phyllosilicates (e.g. chamosite, berthierine) or Fe-rich smectite. If montmorillonite were altered to non-swelling minerals, the expected swelling capacity of the bentonite backfill could be reduced. This paper discuss Fe-rich phyllosilicate mineral stability at the canister-backfill interface using thermodynamic modelling. Estimates of thermodynamic properties were made for Fe-rich clay minerals in order to construct approximate phase-relations for end-member/simplified mineral compositions in activity space. The diagrams (for the system Al O
O -FeO-Fe
-FeO-Fe O
O -MgO-Na
-MgO-Na O-SiO
O-SiO -H
-H O) suggest that if pore waters are supersaturated with magnetite, Fe(II)-rich saponite is the most likely alteration product (if fo
O) suggest that if pore waters are supersaturated with magnetite, Fe(II)-rich saponite is the most likely alteration product (if fo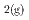 values are significantly lower than magnetite-hematite equilibrium). Therefore, the alteration of montmorillonite may not be detrimental to HLW repositories that include Fe, as long as the swelling behaviour of the Fe-rich smectite produced is maintained. If fo
values are significantly lower than magnetite-hematite equilibrium). Therefore, the alteration of montmorillonite may not be detrimental to HLW repositories that include Fe, as long as the swelling behaviour of the Fe-rich smectite produced is maintained. If fo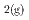 exceeds magnetite-hematite equilibrium, and solutions are saturated with magnetite, berthierine is likely to be more stable than smectite minerals. The alteration of montmorillonite to berthierine could be detrimental to the performance of the repositories.
exceeds magnetite-hematite equilibrium, and solutions are saturated with magnetite, berthierine is likely to be more stable than smectite minerals. The alteration of montmorillonite to berthierine could be detrimental to the performance of the repositories.
Wilson, J.*; Cressey, G.*; Cressey, B.*; Cuadros, J.*; Ragnarsdottir, K. V.*; Savage, D.*; Shibata, Masahiro
Geochimica et Cosmochimica Acta, 70(2), p.323 - 336, 2006/01
Times Cited Count:95 Percentile:85.66(Geochemistry & Geophysics)This work investigates montmorillonite stability in the presence of metal iron, magnetite under hydrothermal conditions. Two series of experiments were conducted. In the first, mixtures of Na-montmorillonite, native Fe, (magnetite, calcite,) and NaCl solutions were reacted at 250 C for about 100 days. In the second, mixtures of Na-montmorillonite, native Fe and FeCl
C for about 100 days. In the second, mixtures of Na-montmorillonite, native Fe and FeCl solutions were reacted at 80
solutions were reacted at 80 250
250 C for about 90 days. In the first series, the starting montmorillonite was transformed to Fe(II)-rich smectite only when the Fe metal was predominantly added. The reaction product was oxidised to Fe(III)-rich form on exposure to air. The expansion of this material on ethylene glycol solvation was much reduced compared to the starting montmorillonite. TEM imaging shows that partial loss of tetrahedral sheets, resulting in adjacent layers becoming H-bonded with a 7 angstrom repeat. Solute activities corresponded to the approximate stability field for Fe(II)-saponite. In the second series, significant smectite alteration was only observed at 250
C for about 90 days. In the first series, the starting montmorillonite was transformed to Fe(II)-rich smectite only when the Fe metal was predominantly added. The reaction product was oxidised to Fe(III)-rich form on exposure to air. The expansion of this material on ethylene glycol solvation was much reduced compared to the starting montmorillonite. TEM imaging shows that partial loss of tetrahedral sheets, resulting in adjacent layers becoming H-bonded with a 7 angstrom repeat. Solute activities corresponded to the approximate stability field for Fe(II)-saponite. In the second series, significant smectite alteration was only observed at 250 C and the product contained a small proportion of a 7
C and the product contained a small proportion of a 7 repeat structure, observable by XRD. In these experiments, solute activities coincide with berthierine.
repeat structure, observable by XRD. In these experiments, solute activities coincide with berthierine.
Oda, Chie; Sasaki, Ryoichi; Honda, Akira; Savage, D.*; Arthur, R. C,*
JNC TN8400 2005-020, 39 Pages, 2005/09
The multiple scenarios of bentonite alteration were ideveloped based on the possible mineralogical changes derived from knowledge of both experiments and observation of natural systems. It was focused that the mineral reaction involving hyperalkaline fluid thermodynamically depends on the variable chemical condition in bentonite buffer and that kinetics is important as well as thermodynamic stability in controlling their occurrence, i.e., the kinetic controls operate to remain metastable minerals over the long term. The mineralogical consequences of the interaction between clays and alkaline fluid are summarized as follows. / -Clay and gel  illite / -Clay and gel
illite / -Clay and gel  metastable zeolite
metastable zeolite  stable zeolite and feldspar / -Clay and gel
stable zeolite and feldspar / -Clay and gel  stable zeolite and feldspar The typical minerals for each category (illite group, metastable zeolite group and stable zeolite and feldspar group) were selected. The scenarios give the range of potential secondary minerals occurring in chemical schemes of the bentonite alteration by cement pore fluids, and thus reasonable assumptions on the simulation of the chemical and mineralogical evolutions of geological disposal system for TRU waste.
stable zeolite and feldspar The typical minerals for each category (illite group, metastable zeolite group and stable zeolite and feldspar group) were selected. The scenarios give the range of potential secondary minerals occurring in chemical schemes of the bentonite alteration by cement pore fluids, and thus reasonable assumptions on the simulation of the chemical and mineralogical evolutions of geological disposal system for TRU waste.
Oda, Chie; Sasaki, Ryoichi; Takase, Hiroyasu*; Savage, D.*; Honda, Akira
Proceedings of International Workshop on Waste Management in Sapporo, p.163 - 165, 2005/08
The uncertainty in our understanding of the precise mineral paragenetic sequences of the interaction of bentonite with hyperalkaline fluids in the long-term still remains. In order to reflect this uncertainty, multiple scenarios of bentonite alteration were investigated based on the possible mineralogical changes derived from knowledge of both experiments and observation of natural systems.
Oda, Chie; Honda, Akira; Sasaki, Ryoichi; Savage, D.*
Extended Abstractof International Workshop on Bentonite-Cement Interaction in Repository Environments, 7 Pages, 2004/04
The possible multiple scenarios of chemical-mineralogical evolution in Bentonite buffer were considered on the basis of the information of both experimental results and observations of natural systems. The range of variable chemical conditions according to each scenario was examined in terms of the potential for locally-attained chemical equilibrium and kinetics. These analyses have been intended to provide reasonable assumptions on simulations using chemistry-transport-hydrology coupling models that aims at estimating the possible evolution of hydraulic condition of bentonite in a TRU waste repository.
Metcalfe, R.*; Savage, D.*; Bath, A. H.*; Walker, C.*
JNC TJ7400 2004-013, 148 Pages, 2004/03
This report describes calculations of the solubilities of elements that are relevant to Performance Assessment in the groundwaters of the Tono area.
Arthur, R. C,*; Savage, D.*; Sasamoto, Hiroshi; Shibata, Masahiro; Yui, Mikazu
JNC TN8400 2000-005, 61 Pages, 2000/01
Kinetic data, including rate constants, reaction orders and activation energies, are compiled for 34 hydrolysis reactions involving feldspars, sheet silicates, zeolites, oxides, pyroxenes and amphiboles, and for similar reactions involving calcite and pyrite. The data are compatible with a rate law consistent with surface reaction control and transition-state theoly, which is incorporated in the geochemieal software package EQ3/6 and GWB. Kinetic data for the reactions noted above are strictly compatible with the transition-state rate law only under far-from-equilibrium conditions. It is possiblethat the data are conceptually consistent with this rate law under both far-from-equilibrium and near-to-equilibrium conditions, but this should be confirmed whenever possible through analysis of original experimental results, Due to limitations in the availability of kinetic data for mineral-water reactions, and in order to simplify evaluations of geochemical models of groundwater evolution, it is convenient to assume local-equilibrium in such models whenever possible. To assess whether this assumption is reasonable, a modeling approach accounting for coupled fluid flow and water-rock interaction is described that can be used to estimate spatial and temporal scale of local equiliblium. The approach is demonstrated for conditions involving groundwater flow in fractures at JNC's Kamaishi in-situ tests site, and is also used to estimate the travel time necessary for oxidizing surface waters to migrate to the level of a HLW repository in crystalline rock. The question of whether local equilibrium is a reasonable assumption must be addressed using an appropriate modeling approach. To be appropriate for conditions at the Kamaishi site using the modeling approach noted above, the fracture fill must closely approximate a porous medium, groundwater flow must be purely advective and diffusion of solutes across the fracture-host rock boundary must not occur. Moreover, the ...
Savage, D.*; Lemke, K.*; Sasamoto, Hiroshi; Shibata, Masahiro; Arthur, R. C,*; Yui, Mikazu
JNC TN8400 2000-004, 30 Pages, 2000/01
Modeling approaches that have been proposed for cement-water system are reviewed in this report, and relevant supporting thsrmodynamic data are compiled. The thermodynamic data include standard molal thermodynamic properties of minerals and related compounds comprising cements, and equilibrium constants for associated hydrolysis reactions. Similar data for minerals that are stable in hyperalkaline geologic environments (e.g., zeolites) are also included because these minerals could be formed as hyperalkaline fluids emanating from cementitious matelials in a repository for radioactive wastes interact with the surrounding host rock. Standard molal properties (i.e., standard molal Gibbs free energies and enthalpies of formation and standard molal entropies), and/or equilibrium constants for associated hydrolysis reactions, are included for. (1)cement minerals and related compounds (Reardon, 1992; Glasser et al., 1999) (2)calcium-silicate hydrate minerals (Sarkar et al., 1982), and (3)zeolites (calorimetric and estimated values from various sources) All these data are accepted at face value, and it is therefore cautioned that the data, considered as a whole, may not be internally consistent. It is also important to note that the accuracy of these data have not been evaluated in the present study. Several models appropriate for cement-water systems have been proposed in recent years. Most are similar in the sense that they represent empirical fits to laboratory data for the CSH gel-water system, and therefore not thermodynamically defensible. An alternative modeling approach based on thermodynamic principles of solid-solution behavior appropriate for CSH gel has recently been proposed, however. It is reviewed in the present study, and evaluated in relation to experimental results obtained by JNC on cement-water interactions. The solid-solution model is based upon a thermodynamically- and structually-justifiable description of CSH gel in terms of a non-ideal ...
Savage, D.*; Arthur, R. C,*; Sasamoto, Hiroshi; Shibata, Masahiro; Yui, Mikazu
JNC TN8400 2000-003, 56 Pages, 2000/01
Geochemical as well as socio-economic issues associated with the selection of potential sites to host a high-level nuclear waste repository have received considerable attention in repository programs in Europe (Belgium, Finland, France, Germany, Spain, Sweden, Switzerland and the U.K.) and North America (Canada and the United States), The objective of the present study is to summarize this international experience with particular emphasis on geochemical properties that factor into the adopted site-selection strategies. Results indicate that the geochemical properties of a site play a subordinate role, at best, to other geotechnical properties in the international site-selection approaches. In countries where geochemical properties are acknowledged in the site-selection approach, requirements are stated qualitatively and tend to focus on associated impacts on the stability of the engineered barrier system and on radionuclide transport. Site geochemical properties that are likely to control the lomg-term stability of geochemical conditions and radionuclide migration behavior are unspecified, however. This non-prescriptive approach may be reasonable for purposes of screeing among potential sites, but a better understanding of site properties that are most important in controlling the long-term geochemical evolution of the site over a range of possible scenarios would enable the potential sites to be ranked in terms of their suitability to host a repository.
Sasamoto, Hiroshi; Yui, Mikazu; Savage, D.*; Bille, B.*
JNC TN8400 99-025, 32 Pages, 1999/06
Groundwater data used for modelling site or repository evolution need to be assessed for their quality and whether they are "fit for purpose", prior to utilization. This report discuss factors and issues which impinge upon the quality of such data. It is recommended that geochemical modelleres : (1)are aware of how groundwater samples were collected, whether during drilling, during hydraulic testing, or thereafter, by in-situ measurement, pumped from boreholes, or by pressurised sampler ; (2)are aware of what procedures (if any) were used to "correct" samples for drill fluid contamination and what errors were associated with those methods ; (3)are aware of whether samples were subject to de-pressurisation during sampling, and whether geochemical modelling techniques were applied to correct the compositions of samples for that process ; (4)request different measures of redox activity (e.g., electrode measurements of Eh, concentrations of different redox-sensitive aqueous species) to be applied to key groundwater samples to investigate the extent of redox equilibrium ; (5)are aware of how groundwater samples were filtered and preserved for off-site analysis ; (6)ensure that adequate methods of groundwater filtration ( 0.1
0.1 m) and chemical analysis are applied to ensure accurate and reproducible analyses for dissolved aluminum at low levels of concentration (generally less than 0.2 mg/L) ; (7)are aware of elemental errors and detection limits in chemical analysis of groundwater samples and assess the quality of groundwater analyses via ion exchange balances and via a comparison of measured and calculated values for total dissolved solids contents. (8)ensure that detailed mineralogical analysis is carried out on rock samples from locations where key groundwater samples have been extracted.
m) and chemical analysis are applied to ensure accurate and reproducible analyses for dissolved aluminum at low levels of concentration (generally less than 0.2 mg/L) ; (7)are aware of elemental errors and detection limits in chemical analysis of groundwater samples and assess the quality of groundwater analyses via ion exchange balances and via a comparison of measured and calculated values for total dissolved solids contents. (8)ensure that detailed mineralogical analysis is carried out on rock samples from locations where key groundwater samples have been extracted.
Oda, Chie; Sasaki, Ryoichi; Arthur, R. C,*; Savage, D.*; Honda, Akira
no journal, ,
Bentonite materials are expected to be used as the main hydraulic barrier and cementitious materials are expected to be used for structure support in low level radioactive waste disposal systems. Interaction between bentonite and hyperalkaline fluids arising from cementitious materials has been recognized as a concern due to the close proximity of these materials in repository designs. The interaction could cause the alteration of mineralogy and associated hydraulic property of bentonite and have a deleterious influence on the function of bentonite as a hydraulic barrier, and may lead to adverse impact on the long-term safety of the repository. Many researches on such interaction have been performed to establish the bentonite alteration scheme, however, uncertainty in our understanding of the precise alteration scheme, especially mineral paragenetic sequences during the alteration, still remains. Therefore, it is important to take such uncertainty into account in estimating how the mineralogy and the hydraulic property of bentonite evolves subject to alteration by hyperalkaline fluids. In this work, multiple scenarios for mineralogical alteration of bentonite was investigated to limit the mineral paragenetic uncertainty and to identify appropriate assumption for numerical simulation of chemical and mineralogical evolution of bentonite under hyperalkaline conditions over the long-term.
Wilson, J.*; Watson, C.*; Benbow, S.*; Savage, D.*; Sasamoto, Hiroshi
no journal, ,
Iron steel overpack will be surrounded by bentonite buffer as a engineering barrier system for the high-level radioactive waste disposal. It is concerned that iron-bentonite interactions result in the alteration of montmorillonite to non-swelling Fe-rich minerals. In the present study, reactive transport modeling of iron-bentonite interface evolution has been conducted to evaluate the long-term behavior of bentonite stability. A number of model cases were produced in order to assess which processes are likely to dominate at iron-bentonite interfaces.
Oda, Chie; Savage, D.*; Benbow, S.*; Watson, C.*; Takase, Hiroyasu*
no journal, ,
An natural clay alteration at Searles Lake, California has been investigated using reaction-transport modelling with the computer code employing time-dependent boundary conditions of sedimentation and fluid composition. The evidence in natural systems can demonstrate that alkaline alteration will be controlled by kinetic-geochemical reaction and transport coupling process, and that advective fluid flow and the salinity of the pore fluid eill be important factors.
Gallardo, A.*; Savage, D.*; Benbow, S.*; Takase, Hiroyasu*; Oda, Chie
no journal, ,
A numerical model was constructed to understand the groundwater flow dynamics and salinity distribution in the Searles Lake, California, a dry and alkaline playa lake, as a natural analogue for processes in a potential geological repository. The result shows that vertical flow would prevail at the center of the lake, and under this condition, salts would be dissolved from shallow evaporitic horizons being redistributed downwards and away from the lake surface. Concentrations up to 220 g/L would occur within an area approximately 6 by 4 km, extending to a depth of 200 m after 200,000 years. Although several uncertainties remain, the model is consistent with many observations, and provides a framework to address geochemical processes in the basin.
Oda, Chie; Yamaguchi, Kohei; Savage, D.*; Benbow, S.*; Watson, C.*; Takase, Hiroyasu*
no journal, ,
Mudstones containing smectite have been altered under mildly alkaline conditions (9  pH
pH  10) at Searles Lake. This natural alteration has been investigated using reaction-transport modelling. These data demonstrate that smectite alteration will be controlled by the kinetics of dissolution-precipitation reaction and the salinity of the pore fluid.
10) at Searles Lake. This natural alteration has been investigated using reaction-transport modelling. These data demonstrate that smectite alteration will be controlled by the kinetics of dissolution-precipitation reaction and the salinity of the pore fluid.
Savage, D.*; Wilson, J.*; Benbow, S.*; Sasamoto, Hiroshi; Oda, Chie; Walker, C.*; Kawama, Daisuke*; Tachi, Yukio
no journal, ,
Safety functions for the clay buffer in a repository for HLW are fulfilled if the presence of montmorillonite is maintained in the long-term. Its transformation to non-swelling minerals (e.g. illite) is addressed in most safety assessments by using semi-empirical kinetic models. However, this approach contrasts with all other near-field geochemical modelling activities that employ complex reaction-transport simulations. Here we investigate the consistency of these two approaches by modelling the montmorillonite to illite transformation in the marine sediment profile penetrated by the Ocean Drilling Program (ODP) Site 1174. Illitisation of smectite at Site 1174 using the semi-empirical approach has been modeled by previous studies, and shown to provide a reasonable match to the gradual change of illite content with depth. In comparison, the initial results of reaction-transport simulations showed rapid (conservative) conversion of montmorillonite to illite. The cause of this rapid conversion appears to be the transformation of amorphous silica to quartz over a similar timescale. Subsequent simulations have focused on alternative mechanisms for mineral growth that may explain the discrepancies between the semi-empirical and reaction-transport approaches.