Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
 complexes of mixed N,O-donor ligands demonstrated on a 10-fold [Eu(TPAMEN)]
complexes of mixed N,O-donor ligands demonstrated on a 10-fold [Eu(TPAMEN)] chelate complex
chelate complexSchnaars, K.; Kaneko, Masashi; Fujisawa, Kiyoshi*
Inorganic Chemistry, 60(4), p.2477 - 2491, 2021/02
Times Cited Count:6 Percentile:59.03(Chemistry, Inorganic & Nuclear)To reduce high-level radiotoxic waste generated by nuclear power plants, highly selective separation agents for minor actinides are mandatory. The mixed N,O-donor ligand  -tetrakis[(6-carboxypyridin-2-yl)methyl]ethylenediamine (H
-tetrakis[(6-carboxypyridin-2-yl)methyl]ethylenediamine (H TPAEN) has shown good performance as a masking agent in Am
TPAEN) has shown good performance as a masking agent in Am /Eu
/Eu separation studies. In this work, we examine whether a decrease in O-donor basicity can promote the M
separation studies. In this work, we examine whether a decrease in O-donor basicity can promote the M -N
-N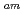 interactions. Therefore, we replace the deprotonated "charged" carboxylic acid groups of TPAEN
interactions. Therefore, we replace the deprotonated "charged" carboxylic acid groups of TPAEN by neutral amide groups and introduce
by neutral amide groups and introduce  -tetrakis[(6-
-tetrakis[(6-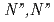 -diethylcarbamoylpyridin-2-yl)methyl]ethylenediamine (TPAMEN) as a new ligand. TPAMEN was crystallized with Eu(OTf)
-diethylcarbamoylpyridin-2-yl)methyl]ethylenediamine (TPAMEN) as a new ligand. TPAMEN was crystallized with Eu(OTf) and Eu(NO
and Eu(NO )
) 6H
6H O to form positively charged 1:1 [Eu(TPAMEN)]
O to form positively charged 1:1 [Eu(TPAMEN)] complexes in the solid state. Alterations in the M-O/N bond distances are compared to [Eu(TPAEN)]
complexes in the solid state. Alterations in the M-O/N bond distances are compared to [Eu(TPAEN)] and investigated by DFT calculations to expose the differences in charge/energy density distributions at europium(III) and the donor functionalities of the TPAEN
and investigated by DFT calculations to expose the differences in charge/energy density distributions at europium(III) and the donor functionalities of the TPAEN and TPAMEN. On the basis of estimations of the bond orders, atomic charges spin populations, and density of states in the Eu and potential Am and Cm complexes, the specific contributions of the donor-metal interaction are analyzed. The prediction of complex formation energy differences for the [M(TPAEN)]
and TPAMEN. On the basis of estimations of the bond orders, atomic charges spin populations, and density of states in the Eu and potential Am and Cm complexes, the specific contributions of the donor-metal interaction are analyzed. The prediction of complex formation energy differences for the [M(TPAEN)] and [M(TPAMEN)]
and [M(TPAMEN)] (M
(M = Eu
= Eu , Am
, Am ) complexes provide an outlook on the potential performance of TPAMEN in Am
) complexes provide an outlook on the potential performance of TPAMEN in Am /Eu
/Eu separation.
separation.
Kusaka, Ryoji; Schnaars, K.; Watanabe, Masayuki
no journal, ,
To unveil the extraction mechanism of metal elements from the aqueous phase to the organic phase, we have recently suggested that it is useful to clarify the structure and the property of the metal complexes formed on the surface of aqueous solutions dissolving metal ion and extractant (ligand). In the present study, in order to reveal the mechanism of the solvent extraction of lanthanides by di-(2-ethylhexyl)phosphoric acid (HDEHP), we measured vibrational spectra of lanthanides-HDEHP complexes on the aqueous solutions in the P=O stretch region with vibrational sum frequency generation (VSFG) spectroscopy, and investigated the structure and properties of the complexes on the surface of the aqueous solutions.