Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Kumada, Takayuki; Nakagawa, Hiroshi; Miura, Daisuke; Sekine, Yurina; Motokawa, Ryuhei; Hiroi, Kosuke; Inamura, Yasuhiro; Oku, Takayuki; Oishi, Kazuki*; Morikawa, Toshiaki*; et al.
Journal of Physical Chemistry Letters (Internet), 14(34), p.7638 - 7643, 2023/08
Times Cited Count:0 Percentile:0.01(Chemistry, Physical)The structure of nano-ice crystals in rapidly frozen glucose solution was elucidated by using spin-contrast-variation small-angle neutron scattering, which distinguishes the nano-ice crystal signal from the frozen amorphous solution signal by the polarization-dependent neutron scattering. The analysis revealed that the nano-ice crystals form a planar structure with a diameter exceeding tens of nanometers and a thickness of 1 nm, which is close to the critical nucleation size. This result suggests that the glucose molecules are preferentially bound to a specific face of nano-ice crystals, and then block the crystal growth perpendicular to that face.
Miura, Daisuke*; Kumada, Takayuki; Sekine, Yurina; Motokawa, Ryuhei; Nakagawa, Hiroshi; Oba, Yojiro; Ohara, Takashi; Takata, Shinichi; Hiroi, Kosuke; Morikawa, Toshiaki*; et al.
Journal of Applied Crystallography, 54(2), p.454 - 460, 2021/04
Times Cited Count:1 Percentile:17.63(Chemistry, Multidisciplinary)We developed a spin-contrast-variation neutron powder diffractometry technique that extracts the structure factor of hydrogen atoms, namely, the contribution of hydrogen atoms to a crystal structure factor. Crystals of L-glutamic acid were dispersed in a dpolystyrene matrix containing 4-methacryloyloxy-2,2,6,6,-tetramethyl-1-piperidinyloxy (TEMPO methacrylate) to polarize their proton spins dynamically. The intensities of the diffraction peaks of the sample changed according to the proton polarization, and the structure factor of the hydrogen atoms was extracted from the proton-polarization dependent intensities. This technique is expected to enable analyses of the structures of hydrogen-containing materials that are difficult to determine with conventional powder diffractometry.
Tanaka, Kumiko; Hirata, Masaru; Sekine, Rika*
Journal of Nuclear and Radiochemical Sciences, 5(2), p.27 - 31, 2004/12
The relativistic discrete-variational Dirac-Fock-Slater (DV-DFS) method was performed to investigate the electronic structure of MgO doped with Pu or Am atoms. The differences between these systems, in particular, the participation of d-electrons and f-electrons in chemical bonding, were clarified by calculating their electronic structures. Substitution of actinide atoms was found to result in the effective charges of MgO becoming smaller, with a large charge transfer occurring as far as the second layer. It was also found that the bonding feature between the center atom and the surrounding oxygen atoms was extended to lower energy in the case of actinide (An) substituted systems. Moreover, the bonding characteristics were assigned; a bonding interaction for An6d-O2p and an anti-bonding for An5f-O2p near the HOMO level. These complex effects were found to dominate the strength of the covalent bonding between MgO and actinide atoms.
 -uranium metal;
-uranium metal;  -uranium alloy with 3d transition metals
-uranium alloy with 3d transition metalsKurihara, Masayoshi*; Hirata, Masaru; Sekine, Rika*; Onoe, Jun*; Nakamatsu, Hirohide*
Journal of Nuclear Materials, 326(2-3), p.75 - 79, 2004/03
Times Cited Count:12 Percentile:61.53(Materials Science, Multidisciplinary)We have investigated the alloying behavior of g-uranium with 3d transition metals (TMs) using the relativistic discrete-variational Dirac-Fock-Slater (DV-DFS) method. The d-orbital energy (Md) as an alloying parameter well reproduces the alloying behavior of g- uranium metal with TMs: (1) in the case of a large Md value (Ti,V,Cr), the solubility of these TM elements in g-uranium becomes large; (2) in the case of a middle Md value (Mn,Fe,Co), the tendency to form a uranium intermetallic compound with these elements becomes stronger; (3) in the case of a small Md value (Cu), the alloying element is insoluble in g-uranium. The alloying behavior of g-uranium with TMs is also discussed in terms of other parameters such as electronegativity and metallic radius.
Hirata, Masaru; Bastug, T.*; Tachimori, Shoichi; Sekine, Rika*; Onoe, Jun*; Nakamatsu, Hirohide*
Advances in Quantum Chemistry, Volume 37, p.325 - 333, 2001/00
no abstracts in English
Hirata, Masaru; Tachimori, Shoichi; Sekine, Rika*; Onoe, Jun*; Nakamatsu, Hirohide*
Advances in Quantum Chemistry, Volume 37, p.335 - 351, 2001/00
no abstracts in English
 -uranium metal
-uranium metalKurihara, Masayoshi*; Hirata, Masaru; Sekine, Rika*; Onoe, Jun*; Nakamatsu, Hirohide*
Journal of Nuclear Materials, 281(2-3), p.140 - 145, 2000/10
Times Cited Count:3 Percentile:26.42(Materials Science, Multidisciplinary)no abstracts in English
Hirata, Masaru; Bastug, T.*; Sekine, Rika*; Onoe, Jun*; Nakamatsu, Hirohide*; Mukoyama, Takeshi*
JAERI-Review 99-008, 29 Pages, 1999/03
no abstracts in English
Kurihara, Masayoshi*; Hirata, Masaru; Sekine, Rika*; Onoe, Jun*; Nakamatsu, Hirohide*; Mukoyama, Takeshi*; *
Journal of Alloys and Compounds, 283, p.128 - 132, 1999/00
Times Cited Count:10 Percentile:59.64(Chemistry, Physical)no abstracts in English
Hirata, Masaru; Sekine, Rika*; Onoe, Jun*; Nakamatsu, Hirohide*; Mukoyama, Takeshi*; *; Tachimori, Shoichi
Journal of Alloys and Compounds, 271-273, p.128 - 132, 1998/00
Times Cited Count:8 Percentile:52.63(Chemistry, Physical)no abstracts in English
 (NO
(NO )
)
 2H
2H O
OHirata, Masaru; *; Sekine, Rika*; Onoe, Jun*; Nakamatsu, Hirohide*; Mukoyama, Takeshi*; *; *
Journal of Electron Spectroscopy and Related Phenomena, 83(1), p.59 - 64, 1997/00
Times Cited Count:19 Percentile:63.17(Spectroscopy)no abstracts in English
 state in
state in 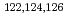 Ba
BaMorikawa, Tsuneyasu*; Oshima, Masumi; Sekine, Toshiaki; ; ; ; *; Shibata, Michihiro; Taniguchi, A.*
Physical Review C, 46(1), p.R6 - R9, 1992/07
Times Cited Count:23 Percentile:75.77(Physics, Nuclear)no abstracts in English
Oshima, Masumi; Sekine, Toshiaki; ; ; *; Morikawa, Tsuneyasu*;
Nuclear Instruments and Methods in Physics Research B, 70, p.241 - 244, 1992/00
Times Cited Count:7 Percentile:60.99(Instruments & Instrumentation)no abstracts in English
Kumada, Takayuki; Nakagawa, Hiroshi; Miura, Daisuke*; Sekine, Yurina; Motokawa, Ryuhei; Hiroi, Kosuke; Inamura, Yasuhiro; Oku, Takayuki; Oishi, Kazuki*; Morikawa, Toshiaki*; et al.
no journal, ,
The structure of nano-ice crystals in rapidly frozen sugar solution was elucidated by using spin-contrast-variation small-angle neutron scattering, which distinguishes the nano-ice crystal signal from the frozen amorphous solution signal by the polarization-dependent neutron scattering. The analysis revealed that the nano-ice crystals form a planar structure with a diameter exceeding tens of nanometers and a thickness of 1 nm, which is close to the critical nucleation size. This result suggests that the sugar molecules are preferentially bound to a specific face of nano-ice crystals, and then block the crystal growth perpendicular to that face.