Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
 binding site of a
binding site of a  -lactamase from the halophile by anomalous X-ray diffraction
-lactamase from the halophile by anomalous X-ray diffractionArai, Shigeki; Shibazaki, Chie; Shimizu, Rumi; Adachi, Motoyasu; Tamada, Taro; Tokunaga, Hiroko*; Ishibashi, Matsujiro*; Tokunaga, Masao*; Kuroki, Ryota
Kyushu Shinkurotoronko Kenkyu Senta Nempo, 2014, p.17 - 19, 2016/03
no abstracts in English
Hiromoto, Takeshi; Adachi, Motoyasu; Shibazaki, Chie; Schrader, T. E.*; Ostermann, A.*; Kuroki, Ryota
JPS Conference Proceedings (Internet), 8, p.033003_1 - 033003_6, 2015/09
T4 phage lysozyme (T4L) is an endoacetylmuramidase that degrades the murein of the bacterial cell wall by cleaving the  -1,4-glycosidic bond between
-1,4-glycosidic bond between  -acetylmuramic acid and
-acetylmuramic acid and  -acetylglucosamine. We previously reported that the substitution of the catalytic Thr26 with the nucleophilic His converts the wild-type (WT) T4L from an inverting to a retaining glycosidase, in which the
-acetylglucosamine. We previously reported that the substitution of the catalytic Thr26 with the nucleophilic His converts the wild-type (WT) T4L from an inverting to a retaining glycosidase, in which the  -configuration of the substrate is retained in the product. We also found that the Thr26His (T26H) mutant can catalyze a transglycosylation reaction more effectively than hydrolysis, although the WT-T4L has no transglycosidase activity. To clarify the role of the substituted His26 in transglycosylation and to investigate its relationship to the neighboring acidic residue Asp20 using neutron crystallography, a perdeuterated recombinant protein of the T26H mutant (d-T26H) was prepared for crystallization. The perdeuterated form was produced in
-configuration of the substrate is retained in the product. We also found that the Thr26His (T26H) mutant can catalyze a transglycosylation reaction more effectively than hydrolysis, although the WT-T4L has no transglycosidase activity. To clarify the role of the substituted His26 in transglycosylation and to investigate its relationship to the neighboring acidic residue Asp20 using neutron crystallography, a perdeuterated recombinant protein of the T26H mutant (d-T26H) was prepared for crystallization. The perdeuterated form was produced in  cells cultured in deuterated-rich media. After purification, the d-T26H mutant was crystallized under deuterated conditions and grown to a volume of 0.12 mm
cells cultured in deuterated-rich media. After purification, the d-T26H mutant was crystallized under deuterated conditions and grown to a volume of 0.12 mm using a macroseeding technique. A preliminary neutron-diffraction experiment at 100 K at the FRM II research reactor (Munich, Germany) gave diffraction spots of up to 2.8
using a macroseeding technique. A preliminary neutron-diffraction experiment at 100 K at the FRM II research reactor (Munich, Germany) gave diffraction spots of up to 2.8  resolution after a 1.5 h exposure.
resolution after a 1.5 h exposure.
 -lactamase from the moderate halophile
-lactamase from the moderate halophile  sp.560 and the discovery of a Cs
sp.560 and the discovery of a Cs -selective binding site
-selective binding siteArai, Shigeki; Yonezawa, Yasushi*; Okazaki, Nobuo*; Matsumoto, Fumiko*; Shibazaki, Chie; Shimizu, Rumi; Yamada, Mitsugu*; Adachi, Motoyasu; Tamada, Taro; Kawamoto, Masahide*; et al.
Acta Crystallographica Section D, 71(3), p.541 - 554, 2015/03
Times Cited Count:7 Percentile:50.76(Biochemical Research Methods)The crystal structure of halophilic  -lactamase from
-lactamase from  sp.560 (HaBLA) was determined using X-ray crystallography. Moreover, the locations of bound Sr
sp.560 (HaBLA) was determined using X-ray crystallography. Moreover, the locations of bound Sr and Cs
and Cs ions were identified by anomalous X-ray diffraction. The location of one Cs
ions were identified by anomalous X-ray diffraction. The location of one Cs specific binding site was identified on HaBLA even in the presence of 9-fold molar excess of Na
specific binding site was identified on HaBLA even in the presence of 9-fold molar excess of Na (90 mM Na
(90 mM Na /10 mM Cs
/10 mM Cs ). This Cs
). This Cs binding site is formed by two main-chain O atoms and an aromatic ring of a side chain of Trp. An aromatic ring of Trp interacts with Cs
binding site is formed by two main-chain O atoms and an aromatic ring of a side chain of Trp. An aromatic ring of Trp interacts with Cs by the cation-
by the cation- interaction. The observation of a selective and high-affinity Cs
interaction. The observation of a selective and high-affinity Cs binding site provides important information that is useful for designing artificial Cs
binding site provides important information that is useful for designing artificial Cs binding sites useful in bioremediation of radioactive isotopes.
binding sites useful in bioremediation of radioactive isotopes.
Shibazaki, Chie; Adachi, Motoyasu; Kuroki, Ryota
no journal, ,
For elucidation of the molecular recognition and catalytic mechanism of biomolecules, not only the detailed molecular structure, but also the hydration structure of the catalytic site and the exact position of hydrogen atoms are important. Collaborative use of both neutron and X-ray crystallography is a powerful methodology to obtain this information. In such experiments, high quality single crystals with large volume enable us to obtain high resolution X-ray diffraction by reducing the effect of X-ray damage, and improve neutron diffraction data by increasing diffraction intensity. Two different approaches have been attempted to obtain large crystals. One is the periodic addition of protein solution to the mother liquor, and the other is to increase the volume of mother liquor. We report the result of increase of the mother liquor volume after focusing on additives and pH to control the nucleation of protein crystals using several proteins.
Shibazaki, Chie; Adachi, Motoyasu; Shimizu, Rumi; Kuroki, Ryota
no journal, ,
Casein kinase 2(CK2) is one of the ubiquitous Ser/Thr kinases and is involved in the cell cycle and the survival and proliferation of cells. CK2 is a heterotetrameric structure comprising two  - or
- or  -subunits. In order to understand the biological function of the alpha catalytic subunit of CK2 (CK2
-subunits. In order to understand the biological function of the alpha catalytic subunit of CK2 (CK2 ), we aim to analyze the structure of CK2
), we aim to analyze the structure of CK2 including information of the hydrogen and hydrating water molecule by neutron crystallography. The gene coding CK2
including information of the hydrogen and hydrating water molecule by neutron crystallography. The gene coding CK2 was inserted into pET24a and expressed in
was inserted into pET24a and expressed in 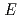 . coli strain BL21DE3, in which the mobile region (330-335) and chemically reactive thiols (Cys147 and Cys220) were removed by amino acid mutation. A total of 150 mg protein was obtained from a 6 L culture, and was used for crystallization trials. The preparation of large crystals was performed using a macro seeding method. Finally, a large crystal with a volume of approximately 2 mm
. coli strain BL21DE3, in which the mobile region (330-335) and chemically reactive thiols (Cys147 and Cys220) were removed by amino acid mutation. A total of 150 mg protein was obtained from a 6 L culture, and was used for crystallization trials. The preparation of large crystals was performed using a macro seeding method. Finally, a large crystal with a volume of approximately 2 mm was reproducibly obtained. The crystal was dialyzed in the deuterated reagent and deuterium water, and the preliminary neutron diffraction experiments were carried out at neutron beam line BioDIFF in research reactor FRM-II in Technical University of Munich. The diffraction was successfully observed greater than 1.9
was reproducibly obtained. The crystal was dialyzed in the deuterated reagent and deuterium water, and the preliminary neutron diffraction experiments were carried out at neutron beam line BioDIFF in research reactor FRM-II in Technical University of Munich. The diffraction was successfully observed greater than 1.9  resolution.
resolution.
Hiromoto, Takeshi; Adachi, Motoyasu; Shibazaki, Chie; Kuroki, Ryota
no journal, ,
T4 phage lysozyme (T4L) is an endoacetylmuramidase that degrades the murein of the bacterial cell wall by cleavage of the  -1,4-glycosidic bond between
-1,4-glycosidic bond between  -acetylmuramic acid and
-acetylmuramic acid and  -acetylglucosamine. We previously reported that the substitution of the catalytic Thr26 to the nucleophilic His converts the wild type (WT) T4L from an inverting to a retaining glycosidase, in which the
-acetylglucosamine. We previously reported that the substitution of the catalytic Thr26 to the nucleophilic His converts the wild type (WT) T4L from an inverting to a retaining glycosidase, in which the  -configuration of the substrate is retained in the product. It was also found that the Thr26His mutant T4L can catalyze the transglycosylation reaction more effectively than hydrolysis although the WT T4L has no transglycosidase activity. To clarify the role of the substituted His26 on transglycosylation and its relationship to the neighboring acidic residue Asp20 by neutron crystallography, the perdeuterated recombinant proteins of the WT and Thr26His mutant T4L were prepared for crystallization in this study. The perdeuterated forms were produced in
-configuration of the substrate is retained in the product. It was also found that the Thr26His mutant T4L can catalyze the transglycosylation reaction more effectively than hydrolysis although the WT T4L has no transglycosidase activity. To clarify the role of the substituted His26 on transglycosylation and its relationship to the neighboring acidic residue Asp20 by neutron crystallography, the perdeuterated recombinant proteins of the WT and Thr26His mutant T4L were prepared for crystallization in this study. The perdeuterated forms were produced in  cells cultured in deuterated rich media. After purification, macroseeding was performed to grow large crystals by transferring individual crystals to hanging drops. A crystal of Thr26His mutant T4L with a volume of 0.1 mm
cells cultured in deuterated rich media. After purification, macroseeding was performed to grow large crystals by transferring individual crystals to hanging drops. A crystal of Thr26His mutant T4L with a volume of 0.1 mm was grown after one month. Preliminary neutron-diffraction experiment at the research reactor FRM-II (Munich, Germany) at 100 K gave diffraction spots beyond 2.5
was grown after one month. Preliminary neutron-diffraction experiment at the research reactor FRM-II (Munich, Germany) at 100 K gave diffraction spots beyond 2.5  resolution for 1.5 hour exposure.
resolution for 1.5 hour exposure.
Adachi, Motoyasu; Shibazaki, Chie; Arai, Shigeki; Shimizu, Rumi; Kuroki, Ryota
no journal, ,
HIV protease-I (HIV-PR) is an important drug target protein for AIDS. Since HIV-PR has a homo dimeric structure, the contribution of each monomer on its structure and function is indistinguishable. To solve this problem, a single chain derivative of HIV-PR (sc-HIV-PR) was designed. The gene coding sc-HIV-PR, in which a linker comprising glycyl-analine was placed between the C-terminal and N-terminal of each HIV-PR, was expressed in E. coli. The inactive form in inclusion bodies was successfully refolded to its active form as reported previously. The tertiary structure of sc-HIV-PR with KNI272 was determined to 1.3  resolution by X-ray crystallography. The structure of scHIV-PR was confirmed to be essentially equivalent to the original dimer form of HIV-PR with a RMSD of less than 0.3
resolution by X-ray crystallography. The structure of scHIV-PR was confirmed to be essentially equivalent to the original dimer form of HIV-PR with a RMSD of less than 0.3  including the structure of active site. This result indicates that the single chain derivatization of HIV-PR was successful and the inserted linker did not affect the overall structure of HIV-PR.
including the structure of active site. This result indicates that the single chain derivatization of HIV-PR was successful and the inserted linker did not affect the overall structure of HIV-PR.
Shibazaki, Chie; Adachi, Motoyasu; Hiromoto, Takeshi; Shimizu, Rumi; Kuroki, Ryota
no journal, ,
Casein kinase 2 (CK2) is one of the ubiquitous Ser/Thr kinases and is involved in the cell cycle and the survival and proliferation of cells. CK2 is a heterotetrameric structure comprising two  - or
- or  -subunits and two regulatory
-subunits and two regulatory  -subunits. In order to understand the biological function of the alpha catalytic subunit of CK2
-subunits. In order to understand the biological function of the alpha catalytic subunit of CK2 , we aim to analyze the structure of CK2
, we aim to analyze the structure of CK2 including information of the hydrogen and hydrating water molecule by neutron crystallography. The gene coding CK2
including information of the hydrogen and hydrating water molecule by neutron crystallography. The gene coding CK2 was inserted into pET24a and expressed in E. coli strain BL21DE3, in which the mobile region and chemically reactive thiols were removed by amino acid mutation. A total of 150 mg protein was obtained from a 6 L culture, and was used for crystallization trials. The preparation of large crystals was performed using a macro seeding method specially developed for CK2
was inserted into pET24a and expressed in E. coli strain BL21DE3, in which the mobile region and chemically reactive thiols were removed by amino acid mutation. A total of 150 mg protein was obtained from a 6 L culture, and was used for crystallization trials. The preparation of large crystals was performed using a macro seeding method specially developed for CK2 . Finally, a large crystal with a volume of approximately 2 mm
. Finally, a large crystal with a volume of approximately 2 mm was reproducibly obtained. From the X-ray diffraction study, we confirmed that the crystals obtained diffracted to approx. 1
was reproducibly obtained. From the X-ray diffraction study, we confirmed that the crystals obtained diffracted to approx. 1  resolution at 100 K after soaking the crystal into the deuterated cryo protectant. The neutron diffraction data collection is planned to obtain a high resolution neutron structure of CK2
resolution at 100 K after soaking the crystal into the deuterated cryo protectant. The neutron diffraction data collection is planned to obtain a high resolution neutron structure of CK2 .
.
Shimizu, Rumi; Hiromoto, Takeshi; Adachi, Motoyasu; Shibazaki, Chie; Kuroki, Ryota
no journal, ,
no abstracts in English
Hiromoto, Takeshi; Shimizu, Rumi; Adachi, Motoyasu; Shibazaki, Chie; Kuroki, Ryota
no journal, ,
no abstracts in English
Shibazaki, Chie; Adachi, Motoyasu; Hiromoto, Takeshi; Shimizu, Rumi; Kuroki, Ryota
no journal, ,
Casein kinase 2(CK2) is one of the ubiquitous Ser/Thr kinases and is involved in the cell cycle and the survival and proliferation of cells. In order to understand the biological function of the alpha catalytic subunit of CK2 (CK2a), we aim to analyze the structure of CK2a including information of the hydrogen and hydrating water molecule by neutron crystallography. The gene coding CK2a was expressed in E. coli, in which the mobile region and chemically reactive thiols were removed by amino acid mutation. The preparation of large crystals with inhibitor (Emodin and CX4945) was performed using a macro seeding method. Finally, large crystals with a volume of approximately 2 mm were reproducibly obtained. The crystals were dialyzed in the deuterated reagent and deuterium water. We have collected high resolution neutron diffraction images of emodin complex and CX-4945 complex at neutron beam line BioDIFF (FRM-II, Munich).
were reproducibly obtained. The crystals were dialyzed in the deuterated reagent and deuterium water. We have collected high resolution neutron diffraction images of emodin complex and CX-4945 complex at neutron beam line BioDIFF (FRM-II, Munich).
Shibazaki, Chie; Kagotani, Yuji*; Adachi, Motoyasu*
no journal, ,
no abstracts in English