Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Toshiyasu, Suguro,; Notoya, Shin; Nishikawa, Yoshiaki*; Nakamura, Ryosuke*; Shibutani, Tomoki; Kuroha, Mitsuhiko; Kamei, Gento
JNC TN8430 2004-004, 27 Pages, 2005/01
In terms of safety assessment of TRU waste disposal, data on plutonium sorption on cementitious materials have been obtained by means of a static batch-type experiment. Because the repository condition will be reducing and be affected by considerable amount of nitrate, the authors carried out the experiments using ordinary portland cement (OPC) under the reducing (Na S
S O
O as added as reductant) and anoxic condition (O
as added as reductant) and anoxic condition (O
 1ppm) and solution of 0 to 0.5 M NaNO
1ppm) and solution of 0 to 0.5 M NaNO . Other experimental conditions are : liquid/solid (L/S) ratios ; 100 and 1000 mL g
. Other experimental conditions are : liquid/solid (L/S) ratios ; 100 and 1000 mL g , Initially aaded plutonium; 2.84
, Initially aaded plutonium; 2.84 10
10 M, Temperature; 25
M, Temperature; 25 5
5 C and Reaction times; 7, 14 and 28 days. The experimental results suggest that distribution coefficient (
C and Reaction times; 7, 14 and 28 days. The experimental results suggest that distribution coefficient (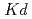 ) ranges 50 to 1000 mL g
) ranges 50 to 1000 mL g in case of L/S=100mL g
in case of L/S=100mL g . Similarly the
. Similarly the 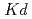 ranges, 100 to 10000 mL g
ranges, 100 to 10000 mL g at L/S=1000mL g
at L/S=1000mL g . These
. These 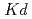 values tend to increase with lapsing reaction time. On the basis of these results, we recommend 50mL g
values tend to increase with lapsing reaction time. On the basis of these results, we recommend 50mL g as a conservative
as a conservative 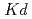 value of plutonium on OPC in a TRU waste repository condition.
value of plutonium on OPC in a TRU waste repository condition.
Tachi, Yukio; Shibutani, Tomoki; Sato, Haruo; Yui, Mikazu
Journal of Contaminant Hydrology, 47(2-4), p.171 - 186, 2001/02
Times Cited Count:35 Percentile:66.88(Environmental Sciences)None
Yui, Mikazu; Shibutani, Tomoki; Shibata, Masahiro; Ochs, M.*; Rai, D.*
Radioactivity in the Environment, 1, p.159 - 174, 2001/00
None
Yui, Mikazu; Shibutani, Tomoki
Using Thermodynamic Sorption Models for Guiding Radioelement Distribution Coefficient (Kd) Investigations, 107 Pages, 2001/00
None
Shibutani, Tomoki; Suyama, Tadahiro*; Shibata, Masahiro
JNC TN8410 99-051, 260 Pages, 1999/11
Distribution coefficients of radionuclides on rocks are selected for safety assessment in the "Second Progress Report on Research and Development for the geological disposal of HLW in Japan (H12 Report)". The categonized types of rock are granitic rocks (crystalline and acidic rocks), basaltic rocks (crystalline and basic rocks), psammitic rocks (neogene sedimentary(soft)), and tuffaceous-pelitic rocks (pre-neogene sedimentary rocks (hard)). The types of groundwater are FRHP (fresh reducing high-pH), FRLP (fresh reducing low-pH), SRHP (saline reducing high-pH), SRLP (saline reducing low-pH), MRNP (mixing reducing neutral-pH) and FOHP (fresh oxidizing high-pH) groundwater. The elements to be surveyed are Ni, Se, Zr, Nb, Tc, Pd, Sn, Cs, Sm, Pb, Ra, Ac, Th, Pa, U, Np, Pu, Am and Cm. Distribution coefficients are collected from literatures describing batch sorption experimental results, and are selected under consideration of conservativity.
Shibutani, Tomoki; Kohara, Yukitoshi*; Oda, Chie; Kubota, Mitsuru*; ; Shibata, Masahiro
JNC TN8400 99-066, 75 Pages, 1999/11
The physico-chemical characteristics of purified Na-smectite and protonation/deprotonation behavior of smectite surface in different concentrations of NaCl solutions were studied to identify the reaction mechanism of smectite-water interaction for performance assessment of HLW geological disposal system. The Na-smectite was purified from Kunipia F (obtained from Kunimine Industries Co. Ltd. Japan). In this smectite, small amount of quartz was detected as an impurity by X-ray diffraction. As a result of XRD and chemical analysis of the smectite, it was found that exchangeable sites in smectite inter-layer were occupied by Na+. Cation exchange capacity (CEC) was measured as 1.108meq g by using ammonium acetate. The N
by using ammonium acetate. The N -BET surface area of smectite was 50
-BET surface area of smectite was 50 58m
58m g
g . Protonation/deprotonation behavior of smectite was studied for 0.01, 0.1 and 0.5M NaCl by using titration and back titration method. Total amount of H
. Protonation/deprotonation behavior of smectite was studied for 0.01, 0.1 and 0.5M NaCl by using titration and back titration method. Total amount of H consumption increased with decreasing pH for all NaCl concentrations, and the titration curves in these solutions showed similar trend in the pH range of 6-11. On the other hand, total amount of H
consumption increased with decreasing pH for all NaCl concentrations, and the titration curves in these solutions showed similar trend in the pH range of 6-11. On the other hand, total amount of H consumption increased with decreasing NaCl concentration in the pH range of 2-6. The dominant sorption mechanism of H
consumption increased with decreasing NaCl concentration in the pH range of 2-6. The dominant sorption mechanism of H on smectite was different between pH
on smectite was different between pH 6 and pH
6 and pH 6, and it can be considered that H
6, and it can be considered that H was sorbed on the same site as Na
was sorbed on the same site as Na for pH
for pH 6 and different site from Na
6 and different site from Na for pH
for pH 6. The prediction of protonation/deprotonation behavior of smectite for 0.01, 0.1 and 0.5M NaCl was carried out based on ion exchange and surface complexation models. The sorption sites were assumed as inter-layer and crystal edge site. The site concentrations for ion exchange and surface complexation were calculated from CEC. The reaction constants were consequently calculated by fitting of experimental results as follows. ...
6. The prediction of protonation/deprotonation behavior of smectite for 0.01, 0.1 and 0.5M NaCl was carried out based on ion exchange and surface complexation models. The sorption sites were assumed as inter-layer and crystal edge site. The site concentrations for ion exchange and surface complexation were calculated from CEC. The reaction constants were consequently calculated by fitting of experimental results as follows. ...
Sato, Haruo; ; Shibutani, Tomoki; Yui, Mikazu; ;
Nuclear Technology, 127(2), p.199 - 211, 1999/08
Times Cited Count:28 Percentile:86.67(Nuclear Science & Technology)None
Notoya, Shin; Shibutani, Tomoki; Okazaki, M.*; Inui, Shinichi*; Kuroha, Mitsuhiko; Yui, Mikazu
JAERI-Conf 99-004, p.643 - 653, 1999/03
None
Shibutani, Tomoki; Shibutani, Sanae
PNC TN8410 98-082, 97 Pages, 1998/06
In the performance assessment of geological disposal system of high-evel radioactive waste(HLW), solubility and speciation of radioactive elements are important to estimate their migration behavior from vitrified HLW in engineered and natural barrier system. The solubility and speciation of radioactive elements are calculated by using thermodynamic data and geochemical code, so development of reliable thermodynamic data base is important to built confidence for performance assessment. In this report, reaction constants of plutonium oxidation/reduction, hydrolysis, complexation for carbonate, sulphate, phosphate, nitrate, fluoride and chloride are discussed and selected. For the development of the PNC-TDB, existing literature data were surveyed and realistic thermodynamic data were selected under consideration of chemical properties, chemical analogy of other actinide element and the internal consistency with the hole PNC-TDB. Solubilities of plutonium were studied by several researchers, and these measurement have been compared with geochemical calculation for validation of the PNC-TDB. Calculated results shows that the experimental results can be interpreted by geochemical calculation by using the PNC-TDB.
Shibutani, Tomoki; Shibutani, Sanae; Yui, Mikazu
PNC TN8410 98-052, 18 Pages, 1998/03
In the performance analysis of geological disposal system of high-level radioactive waste (HLW), solubilities of radioactive elements are estimated by thermodynamic calculation. The reliable thermodynamic database (TDB) is needed for solubility estimation. In this report, thermodynamic data for protactinium solid and aqueous species for performance assessment were selected. For the refinement of previous PNC in house thermodynamic database (PNC-TDB), existing literatures data were surveyed and reliable thermodynamic data were selected under consideration of the scientific defensibility and the consistency with the whole PNC-TDB. The estimated solubility using refined PNC-TDB was higher than measured value. We have confirmed the refined data-set of Pa to be conservative for solubility estimation of performance assessment.
Ashida, Takashi; Kohara, Yukitoshi*; Shibutani, Tomoki; Yui, Mikazu
PNC TN8410 98-014, 30 Pages, 1998/03
None
; Shibutani, Tomoki; Sato, Haruo;
Journal of Contaminant Hydrology, 35, p.77 - 89, 1998/00
Times Cited Count:37 Percentile:70.54(Environmental Sciences)None
Sato, Haruo; Yui, Mikazu; Shibutani, Tomoki
Proceedings of Migration '97, 0 Pages, 1997/10
None
; Shibutani, Tomoki; Sato, Haruo; Yui, Mikazu
Proceedings of Migration '97, 0 Pages, 1997/00
None
; Sato, Haruo; Shibutani, Tomoki;
Proceedings of Migration '97, 0 Pages, 1997/00
None
Shibutani, Tomoki; Yui, Mikazu; Kuroha, Mitsuhiko
Proceedings of Migration '97, 0 Pages, 1996/00
None
 concentration system
concentration systemShibutani, Sanae; Shibutani, Tomoki; Yoshikawa, Hideki; Yui, Mikazu
PNC TN8410 95-204, 24 Pages, 1995/09
None
Shibutani, Tomoki; Nishikawa, Yoshiaki*; Inui, Shinichi*; Uchidate, Nobuyuki*; Yui, Mikazu
PNC TN8410 94-395, 41 Pages, 1994/10
None