Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
 O
O -75MoO
-75MoO binary glasses composed of isolated MoO
binary glasses composed of isolated MoO

Masuno, Atsunobu*; Munakata, Sae*; Okamoto, Yoshihiro; Yaji, Toyonari*; Kosugi, Yoshihisa*; Shimakawa, Yuichi*
Inorganic Chemistry, 63(12), p.5701 - 5708, 2024/03
Times Cited Count:2 Percentile:60.73(Chemistry, Inorganic & Nuclear)Transparent and brown La O
O -MoO
-MoO binary glasses were prepared in bulk form using a levitation technique. The glass-forming range was limited, with the primary composition being approximately 25 mol% La
binary glasses were prepared in bulk form using a levitation technique. The glass-forming range was limited, with the primary composition being approximately 25 mol% La O
O . This glass exhibited a clear crystallization at 546
. This glass exhibited a clear crystallization at 546  C, while determining its glass transition temperature was difficult. Notably, despite its amorphous nature, the glass possessed a density and packing density comparable to those of crystalline La
C, while determining its glass transition temperature was difficult. Notably, despite its amorphous nature, the glass possessed a density and packing density comparable to those of crystalline La Mo
Mo O
O . X-ray absorption fine structure and Raman scattering analyses revealed that the glass structure closely resembles La
. X-ray absorption fine structure and Raman scattering analyses revealed that the glass structure closely resembles La Mo
Mo O
O due to the presence of isolated MoO
due to the presence of isolated MoO
 units, whereas disordered atomic arrangement around La atoms was confirmed. The glass demonstrated transparency ranging from 378 to 5500 nm, and the refractive index at 1.0
units, whereas disordered atomic arrangement around La atoms was confirmed. The glass demonstrated transparency ranging from 378 to 5500 nm, and the refractive index at 1.0  m was estimated to be 2.0. The optical bandgap energy was 3.46 eV, which was slightly smaller than that of La
m was estimated to be 2.0. The optical bandgap energy was 3.46 eV, which was slightly smaller than that of La Mo
Mo O
O . Additionally, the glass displayed a transparent region ranging from 6.5 to 8.0
. Additionally, the glass displayed a transparent region ranging from 6.5 to 8.0  m. This occurrence results from the decreased diversity of MoO
m. This occurrence results from the decreased diversity of MoO units and connectivity of Mo-O-Mo, which resulted in the reduced overlap of multiphonon absorption. This glass formation, with its departure from conventional glass-forming rules, resulted in distinctive glasses with crystal-like atomic arrangements.
units and connectivity of Mo-O-Mo, which resulted in the reduced overlap of multiphonon absorption. This glass formation, with its departure from conventional glass-forming rules, resulted in distinctive glasses with crystal-like atomic arrangements.
Kosugi, Yoshihisa*; Goto, Matato*; Tan, Z.*; Kan, Daisuke*; Isobe, Masahiko*; Yoshii, Kenji; Mizumaki, Masaichiro*; Fujita, Asaya*; Takagi, Hidenori*; Shimakawa, Yuichi*
Scientific Reports (Internet), 11(1), p.12682_1 - 12682_8, 2021/06
Times Cited Count:7 Percentile:41.33(Multidisciplinary Sciences)Caloric effects of solids provide more efficient and environment-friendly innovative refrigeration systems compared to the widely-used conventional vapor compressive cooling systems. Exploring novel caloric materials is challenging but critically important in developing future technologies. Here we discovered that the quadruple perovskite structure ferrimagnet BiCu Cr
Cr O
O shows a large multicaloric effect at the first-order charge transition occurred around 190 K. Large latent heat and the corresponding isothermal entropy changes 28.2 J K
shows a large multicaloric effect at the first-order charge transition occurred around 190 K. Large latent heat and the corresponding isothermal entropy changes 28.2 J K kg
kg can be fully utilized by applying both magnetic fields (magnetocaloric effect) and pressure (barocaloric effect). Adiabatic temperature changes reach 3.9 K for the 50 kOe magnetic field and 4.8 K for the 4.9 kbar pressure, and thus highly efficient thermal controls are achieved by multiple ways.
can be fully utilized by applying both magnetic fields (magnetocaloric effect) and pressure (barocaloric effect). Adiabatic temperature changes reach 3.9 K for the 50 kOe magnetic field and 4.8 K for the 4.9 kbar pressure, and thus highly efficient thermal controls are achieved by multiple ways.
 studied by X-ray spectroscopy
studied by X-ray spectroscopyMizumaki, Masaichiro*; Fujii, Hitoshi*; Yoshii, Kenji; Hayashi, Naoaki*; Saito, Takashi*; Shimakawa, Yuichi*; Uozumi, Takayuki*; Takano, Mikio*
Physica Status Solidi (C), 12(6), p.818 - 821, 2015/06
Times Cited Count:11 Percentile:94.66(Physics, Condensed Matter)We investigated the electronic structure of BaFeO by using HAXPES and XAS measurements and first principle studies. The experimental and theoretical results indicated that BaFeO
by using HAXPES and XAS measurements and first principle studies. The experimental and theoretical results indicated that BaFeO is a negative charge transfer compound. We concluded that the on-site Coulomb energy and the strong hybridization between Fe-3d and O-2p orbitals play a very important role of emergence of negative charge transfer. And we found the new structure in the Fe-2p XPS spectrum and concluded this structure is originated from non-local screening.
is a negative charge transfer compound. We concluded that the on-site Coulomb energy and the strong hybridization between Fe-3d and O-2p orbitals play a very important role of emergence of negative charge transfer. And we found the new structure in the Fe-2p XPS spectrum and concluded this structure is originated from non-local screening.

Mizumaki, Masaichiro*; Yoshii, Kenji; Hayashi, Naoaki*; Saito, Takashi*; Shimakawa, Yuichi*; Takano, Mikio*
Journal of Applied Physics, 114(7), p.073901_1 - 073901_6, 2013/08
Times Cited Count:23 Percentile:62.72(Physics, Applied)We have investigated the magnetocaloric effect (MCE) of a perovskite oxide, BaFeO , that shows ferromagnetism by the aid of a small external field of about 0.3 T below the Curie temperature T
, that shows ferromagnetism by the aid of a small external field of about 0.3 T below the Curie temperature T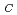 = 111 K. The magnetization is found to change almost reversibly in both field and temperature cycles. Hence, magnetic and thermal hysteretic losses are negligibly low during refrigeration, a property that is suitable for application. The reversible MCE is likely to arise from the absence of an orbital magnetic moment of Fe ions which are essentially in the Fe
= 111 K. The magnetization is found to change almost reversibly in both field and temperature cycles. Hence, magnetic and thermal hysteretic losses are negligibly low during refrigeration, a property that is suitable for application. The reversible MCE is likely to arise from the absence of an orbital magnetic moment of Fe ions which are essentially in the Fe L state (L: ligand hole). The magnetic entropy change and refrigerant capacity near the T
L state (L: ligand hole). The magnetic entropy change and refrigerant capacity near the T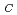 are about 5.8 J kg
are about 5.8 J kg K
K and about 172 J kg
and about 172 J kg , respectively, both of which are comparable to those of ferromagnetic perovskite manganites. As BaFeO
, respectively, both of which are comparable to those of ferromagnetic perovskite manganites. As BaFeO contains no rare metals and is stable against corrosion, the material can be regarded as a candidate refrigerant material.
contains no rare metals and is stable against corrosion, the material can be regarded as a candidate refrigerant material.
Long, Y.-W.*; Kawakami, Takateru*; Chen, W.-T.*; Saito, Takashi*; Watanuki, Tetsu; Nakakura, Yuta*; Liu, Q.-Q.*; Jin, C.-Q.*; Shimakawa, Yuichi*
Chemistry of Materials, 24(11), p.2235 - 2239, 2012/06
Times Cited Count:39 Percentile:71.02(Chemistry, Physical)An A-site ordered perovskite-structure oxide, LaCu Fe
Fe O
O , shows unusual intermetallic charge transfer between the A-site Cu and the B-site Fe ions. Like temperature, pressure also induces the intermetallic charge transfer at room temperature and the compound changes from low-pressure LaCu
, shows unusual intermetallic charge transfer between the A-site Cu and the B-site Fe ions. Like temperature, pressure also induces the intermetallic charge transfer at room temperature and the compound changes from low-pressure LaCu
 Fe
Fe
 O
O to high-pressure LaCu
to high-pressure LaCu
 Fe
Fe
 O
O accompanying with significant volume collapse and as well as unusual softening in bulk modulus. In addition, the material was changed from an antiferromagnetic insulator to a paramagnetic metal transition. Either by physical or chemical (cation substitution) pressure, the charge-transfer transition temperature decreases, and the lower volume phase stabilizes Cu
accompanying with significant volume collapse and as well as unusual softening in bulk modulus. In addition, the material was changed from an antiferromagnetic insulator to a paramagnetic metal transition. Either by physical or chemical (cation substitution) pressure, the charge-transfer transition temperature decreases, and the lower volume phase stabilizes Cu and Fe
and Fe at the A and B sites, respectively.
at the A and B sites, respectively.
 induced by intermetallic charge transfer
induced by intermetallic charge transferAzuma, Masaki*; Chen, W.*; Seki, Hayato*; Czapski, M.*; Olga, S.*; Oka, Kengo*; Mizumaki, Masaichiro*; Watanuki, Tetsu; Ishimatsu, Naoki*; Kawamura, Naomi*; et al.
Nature Communications (Internet), 2, p.347_1 - 347_5, 2011/06
Times Cited Count:397 Percentile:99.15(Multidisciplinary Sciences)no abstracts in English
 Al
Al O
O
Toyama, Takenori*; Saito, Takashi*; Mizumaki, Masaichiro*; Agui, Akane; Shimakawa, Yuichi*
Inorganic Chemistry, 49(5), p.2492 - 2495, 2010/01
Times Cited Count:28 Percentile:73.38(Chemistry, Inorganic & Nuclear)no abstracts in English
Long, Y.*; Saito, Takashi*; Mizumaki, Masaichiro*; Agui, Akane; Shimakawa, Yuichi*
Journal of the American Chemical Society, 131(44), p.16244 - 16247, 2009/10
Times Cited Count:64 Percentile:80.57(Chemistry, Multidisciplinary)A-site-ordered perovskites LaMn Cr
Cr O
O and LaMn
and LaMn Ti
Ti O
O were synthesized under high-pressure and high-temperature conditions. The charge formula of LaMn
were synthesized under high-pressure and high-temperature conditions. The charge formula of LaMn Cr
Cr O
O was found to be LaMn
was found to be LaMn Cr
Cr O
O with Mn
with Mn at the square-coordinated A site. The valence of the A-site Mn ions in LaMnTi
at the square-coordinated A site. The valence of the A-site Mn ions in LaMnTi O
O appeared to be less than +2, and the charge combination in this compound seemed to be LaMnTi
appeared to be less than +2, and the charge combination in this compound seemed to be LaMnTi O
O . Although the square-coordinated A site in A-site-ordered perovskites has been widely known to be occupied by Jahn-Teller active Mn
. Although the square-coordinated A site in A-site-ordered perovskites has been widely known to be occupied by Jahn-Teller active Mn , the present results show that the valence of Mn at the A site can vary from +3 to possible +1.67.
, the present results show that the valence of Mn at the A site can vary from +3 to possible +1.67.
 Mn
Mn O
O (NO
(NO ) oxynitrate comprising
) oxynitrate comprising 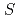 =3/2 honeycomb lattice
=3/2 honeycomb latticeSmirnova, O.*; Azuma, Masaki*; Kumada, Nobuhiro*; Kusano, Yoshihiro*; Matsuda, Masaaki; Shimakawa, Yuichi*; Takei, Takahiro*; Yonesaki, Yoshinori*; Kinomura, Nobukazu*
Journal of the American Chemical Society, 131(23), p.8313 - 8317, 2009/05
Times Cited Count:126 Percentile:91.57(Chemistry, Multidisciplinary)Neutron diffraction and FT-IR analysis revealed that the novel oxynitrate Bi Mn
Mn O
O (NO
(NO ) prepared by hydrothermal synthesis is of a new structural type including flat NO
) prepared by hydrothermal synthesis is of a new structural type including flat NO layers alternating with blocks of two PbSb
layers alternating with blocks of two PbSb O
O -like layers. Mn
-like layers. Mn (
(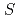 =3/2) forms a regular honeycomb lattice, and magnetic susceptibility data indicated two-dimensional magnetism. Despite its Weiss constant of
=3/2) forms a regular honeycomb lattice, and magnetic susceptibility data indicated two-dimensional magnetism. Despite its Weiss constant of 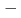 257K, no long-range ordering was observed down to 0.4K because of the magnetic frustration due to the competition between the nearest and the next-nearest antiferromagnetic interactions.
257K, no long-range ordering was observed down to 0.4K because of the magnetic frustration due to the competition between the nearest and the next-nearest antiferromagnetic interactions.
Nojiri, Naoki; Handa, Yuichi*; Shimakawa, Satoshi; Goto, Minoru; Kaneko, Yoshihiko*
Nihon Genshiryoku Gakkai Wabun Rombunshi, 5(3), p.241 - 250, 2006/09
It was shown from the annular core experiment of the HTTR that the discrepancy of excess reactivity between experiment and analysis reached about 3 % Dk/k at maximum. Sensitivity analysis for the annular core of the HTTR was performed to improve the discrepancy. The SRAC code system was used for the core analysis. As the results of the analysis, it was found clearly that the multiplication factor of the annular core is affected by (1) mesh interval in the core diffusion calculation, (2) mesh structure of graphite region in fuel lattice cell and (3) the Benoist's anisotropic diffusion coefficients. The significantly large discrepancy previously reported was reduced down to about 1 % Dk/k by the revised annular core model.

Belik, A. A.*; Iikubo, Satoshi; Kodama, Katsuaki; Igawa, Naoki; Shamoto, Shinichi; Niitaka, Seiji*; Azuma, Masaki*; Shimakawa, Yuichi*; Takano, Mikio*; Izumi, Fujio*; et al.
Chemistry of Materials, 18(3), p.798 - 803, 2006/02
Times Cited Count:292 Percentile:98.59(Chemistry, Physical)The crystal and magnetic structures of polycrystalline BiCoO have been determined by the Rietveldmethod from neutron diffraction data measured at temperatures from 5 to 520 K. BiCoO
have been determined by the Rietveldmethod from neutron diffraction data measured at temperatures from 5 to 520 K. BiCoO (space groupP4mm; Z=1; a=3.72937(7)
(space groupP4mm; Z=1; a=3.72937(7)  and c=4.72382(15)
and c=4.72382(15)  at room temperature; tetragonality c/a=1.267) is isotypic with BaTiO
at room temperature; tetragonality c/a=1.267) is isotypic with BaTiO and PbTiO
and PbTiO in the whole temperature range. BiCoO
in the whole temperature range. BiCoO is an insulator with a Neeltemperature of 470 K. A possible model for antiferromagnetic order is proposed with a propagationvector of k=(1/2, 1/2, 0). In this model, magnetic moments of Co
is an insulator with a Neeltemperature of 470 K. A possible model for antiferromagnetic order is proposed with a propagationvector of k=(1/2, 1/2, 0). In this model, magnetic moments of Co ions are parallel to the c directionand align antiferromagnetically in the ab plane. The antiferromagnetic ab layers stack ferromagneticallyalong the c axis, forming a C-type antiferromagnetic structure. Refined magnetic moments at 5 and 300K are 3.24(2)
ions are parallel to the c directionand align antiferromagnetically in the ab plane. The antiferromagnetic ab layers stack ferromagneticallyalong the c axis, forming a C-type antiferromagnetic structure. Refined magnetic moments at 5 and 300K are 3.24(2)
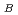 and 2.93(2)
and 2.93(2)
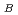 , respectively. The structure refinements revealed no deviation fromstoichiometry in BiCoO
, respectively. The structure refinements revealed no deviation fromstoichiometry in BiCoO . BiCoO
. BiCoO decomposed in air above 720 K to give Co
decomposed in air above 720 K to give Co O
O and sillenite-like Bi
and sillenite-like Bi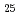 CoO
CoO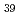 .
.
 B
B O
O (B=Ti,V,Cr)
(B=Ti,V,Cr)Saito, Takashi*; Lon, Y.*; Mizumaki, Masaichiro*; Agui, Akane; Shimakawa, Yuichi*
no journal, ,
no abstracts in English

Mizumaki, Masaichiro*; Agui, Akane; Uozumi, Takayuki*; Inoue, Akira*; Kawai, Masanori*; Ichikawa, Noriya*; Shimakawa, Yuichi*
no journal, ,
no abstracts in English
 by using a soft X-ray emission spectroscopy
by using a soft X-ray emission spectroscopyMizumaki, Masaichiro*; Agui, Akane; Uozumi, Takayuki*; Ichikawa, Noriya*; Shimakawa, Yuichi*
no journal, ,
no abstracts in English

Mizumaki, Masaichiro*; Yoshii, Kenji; Hayashi, Naoaki*; Saito, Takashi*; Shimakawa, Yuichi*; Takeuchi, Yayoi*; Takano, Mikio*
no journal, ,
BaFeO is known to contain the rare valence state of iron, 4+. Although this system has been reported to be an antiferromagnet prepared in the conventional high pressure and high temperature method, the samples synthesized under low temperature oxidization was found to be ferromagnetic. BaFeO
is known to contain the rare valence state of iron, 4+. Although this system has been reported to be an antiferromagnet prepared in the conventional high pressure and high temperature method, the samples synthesized under low temperature oxidization was found to be ferromagnetic. BaFeO shows a Curie temperature of 100 K; however, more details of magnetic properties have not been reported. In this study we report the magnetocaloric effect and the electronic structure of BaFeO
shows a Curie temperature of 100 K; however, more details of magnetic properties have not been reported. In this study we report the magnetocaloric effect and the electronic structure of BaFeO .
.

Mizumaki, Masaichiro*; Agui, Akane; Saito, Takashi*; Azuma, Masaki*; Shimakawa, Yuichi*; Takano, Mikio*; Uozumi, Takayuki*
no journal, ,
no abstracts in English