Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
 -dioctylthiodiglycolamic acid; Effect of S donor on metal extraction
-dioctylthiodiglycolamic acid; Effect of S donor on metal extractionShimojo, Kojiro; Fujiwara, Iori*; Saito, Takumi*; Oshima, Tatsuya*
Analytical Sciences, 40(8), p.1429 - 1436, 2024/08
Times Cited Count:0 Percentile:0.00(Chemistry, Analytical)Extraction ability of  -dioctylthiodiglycolamic acid (T-DODGAA), a soft-base sulfur donor ligand with an amide group and a carboxylic acid connected by a thioether chain, for 56 metal ions have been comprehensively investigated and compared with that of N,N-dioctyldiglycolamic acid (DODGAA) with an etheric oxygen atom, a hard-base donor. The p
-dioctylthiodiglycolamic acid (T-DODGAA), a soft-base sulfur donor ligand with an amide group and a carboxylic acid connected by a thioether chain, for 56 metal ions have been comprehensively investigated and compared with that of N,N-dioctyldiglycolamic acid (DODGAA) with an etheric oxygen atom, a hard-base donor. The p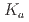 of the thiodiglycolamic acid framework was determined to be 3.71
of the thiodiglycolamic acid framework was determined to be 3.71  0.06 in water (0.1 M LiCl, 25
0.06 in water (0.1 M LiCl, 25 C ) by potentiometric titration, indicating that T-DODGAA is a slightly weaker acid than DODGAA (p
C ) by potentiometric titration, indicating that T-DODGAA is a slightly weaker acid than DODGAA (p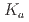 = 3.54
= 3.54  0.03). T-DODGAA can quantitatively extract various metal ions from the 56 metal ions through a proton-exchange reaction. T-DODGAA provided higher extraction performance than DODGAA for Hf(IV), Cr(III), Fe(III), Ni(II), Cu(II), Pd(II), Ag(I), Au(III), Hg(II), Al(III), and Ga(III), especially for soft metal ions. Furthermore, to demonstrate the practical feasibility of T-DODGAA for hydrometallurgy and metal recycling, we performed selective separation tests of rare metal ions such as Sc(III), Ni(II), Co(II), Pd(II), Au(III), In(III), and Ga(II) in metal-mixed extraction systems.
0.03). T-DODGAA can quantitatively extract various metal ions from the 56 metal ions through a proton-exchange reaction. T-DODGAA provided higher extraction performance than DODGAA for Hf(IV), Cr(III), Fe(III), Ni(II), Cu(II), Pd(II), Ag(I), Au(III), Hg(II), Al(III), and Ga(III), especially for soft metal ions. Furthermore, to demonstrate the practical feasibility of T-DODGAA for hydrometallurgy and metal recycling, we performed selective separation tests of rare metal ions such as Sc(III), Ni(II), Co(II), Pd(II), Au(III), In(III), and Ga(II) in metal-mixed extraction systems.
 molecular dynamics simulations reveal the hydration structure of the radium(II) ion
molecular dynamics simulations reveal the hydration structure of the radium(II) ionYamaguchi, Akiko; Nagata, Kojiro*; Kobayashi, Keita; Tanaka, Kazuya; Kobayashi, Toru; Tanida, Hajime; Shimojo, Kojiro; Sekiguchi, Tetsuhiro; Kaneta, Yui; Matsuda, Shohei; et al.
iScience (Internet), 25(8), p.104763_1 - 104763_12, 2022/08
Times Cited Count:17 Percentile:68.06(Multidisciplinary Sciences)no abstracts in English
Shimojo, Kojiro; Fujiwara, Iori*; Oshima, Tatsuya*; Yokoyama, Keiichi; Yaita, Tsuyoshi
Analytical Sciences, 38(7), p.1003 - 1006, 2022/07
Times Cited Count:2 Percentile:13.92(Chemistry, Analytical)Liquid-liquid extraction of lanthanide (Ln) ions was investigated using  -dioctylthiodiglycolamic acid (DOTDGAA), which is a sulfur donor ligand with an amide group and a carboxyl group connected by a thioether chain. The extraction performance and selectivity of DOTDGAA for Ln ions were compared with those of
-dioctylthiodiglycolamic acid (DOTDGAA), which is a sulfur donor ligand with an amide group and a carboxyl group connected by a thioether chain. The extraction performance and selectivity of DOTDGAA for Ln ions were compared with those of  -dioctyldiglycolamic acid (DODGAA), which is also an oxygen donor ligand with a similar chemical structure, to assess the effect of the soft/hard donor atom on Ln separation. DOTDGAA quantitatively extracted all Ln ions while being selective toward light and middle Ln ions, in contrast to the selectivity of DODGAA for heavier Ln ions. Slope analysis demonstrated that the Ln
-dioctyldiglycolamic acid (DODGAA), which is also an oxygen donor ligand with a similar chemical structure, to assess the effect of the soft/hard donor atom on Ln separation. DOTDGAA quantitatively extracted all Ln ions while being selective toward light and middle Ln ions, in contrast to the selectivity of DODGAA for heavier Ln ions. Slope analysis demonstrated that the Ln transfer using DOTDGAA proceeded through a proton-exchange reaction, forming a 1:3 complex, Ln(DOTDGAA)
transfer using DOTDGAA proceeded through a proton-exchange reaction, forming a 1:3 complex, Ln(DOTDGAA) . The back-extraction of Ln ions from the extracting phase was successfully achieved under acidic conditions.
. The back-extraction of Ln ions from the extracting phase was successfully achieved under acidic conditions.
Matsuda, Shohei; Yokoyama, Keiichi; Yaita, Tsuyoshi; Kobayashi, Toru; Kaneta, Yui; Simonnet, M.; Sekiguchi, Tetsuhiro; Honda, Mitsunori; Shimojo, Kojiro; Doi, Reisuke; et al.
Science Advances (Internet), 8(20), p.eabn1991_1 - eabn1991_11, 2022/05
Times Cited Count:8 Percentile:42.69(Multidisciplinary Sciences)no abstracts in English
Yamaguchi, Akiko; Nagata, Kojiro*; Tanaka, Kazuya; Kobayashi, Keita; Kobayashi, Toru; Shimojo, Kojiro; Tanida, Hajime; Sekiguchi, Tetsuhiro; Kaneta, Yui; Matsuda, Shohei; et al.
Hosha Kagaku, (45), p.28 - 30, 2022/03
no abstracts in English
Simonnet, M.; Kobayashi, Toru; Shimojo, Kojiro; Yokoyama, Keiichi; Yaita, Tsuyoshi
Inorganic Chemistry, 60(17), p.13409 - 13418, 2021/09
Times Cited Count:25 Percentile:91.54(Chemistry, Inorganic & Nuclear)Shimojo, Kojiro; Suzuki, Hideya; Yokoyama, Keiichi; Yaita, Tsuyoshi; Ikeda, Atsushi
Analytical Sciences, 36(12), p.1435 - 1437, 2020/12
Times Cited Count:19 Percentile:68.40(Chemistry, Analytical)Liquid-liquid extraction for the removal of pertechnetate ( TcO
TcO
 ) and perrhenate (ReO
) and perrhenate (ReO
 ) is reported using tripodal extractant
) is reported using tripodal extractant 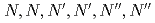 -hexa-
-hexa- -octylnitrilotriacetamide (HONTA) composed of three amide groups and a tertiary amine. The extraction behavior was compared with those using alkyldiamideamines (ADAAM(Oct) and ADAAM(EH)), and the commercial amine-type extractant, trioctylamine (TOA). HONTA quantitatively extracted
-octylnitrilotriacetamide (HONTA) composed of three amide groups and a tertiary amine. The extraction behavior was compared with those using alkyldiamideamines (ADAAM(Oct) and ADAAM(EH)), and the commercial amine-type extractant, trioctylamine (TOA). HONTA quantitatively extracted  TcO
TcO
 and ReO
and ReO
 in the pH range from 1.0 to 2.5 by co-extraction of protons. Extraction performance of extractants was improved in the order of HONTA
in the pH range from 1.0 to 2.5 by co-extraction of protons. Extraction performance of extractants was improved in the order of HONTA  ADAAM(Oct)
ADAAM(Oct)  ADAAM(EH)
ADAAM(EH)  TOA.
TOA.  TcO
TcO
 and ReO
and ReO
 in the extracting phase were successfully stripped using neutral aqueous solutions as the receiving phase, and the extraction ability of HONTA was maintained after five repeated uses.
in the extracting phase were successfully stripped using neutral aqueous solutions as the receiving phase, and the extraction ability of HONTA was maintained after five repeated uses.
Okamura, Hiroyuki; Mizuno, Masayoshi*; Hirayama, Naoki*; Shimojo, Kojiro; Naganawa, Hirochika; Imura, Hisanori*
Industrial & Engineering Chemistry Research, 59(1), p.329 - 340, 2020/01
Times Cited Count:15 Percentile:47.49(Engineering, Chemical)The synergistic ionic-liquid extraction and extraction equilibrium of lanthanoid(III) (Ln(III)) ions have been investigated using 2-thenoyltrifluoroacetone (Htta) and trioctylphosphine oxide (TOPO) in the ionic liquid 1-butyl-3-methylimidazolium bis(trifluoromethanesulfonyl)imide ([C mim][Tf
mim][Tf N]). The selective synergistic effect for heavier Ln(III) ions was found using a combination of Htta and TOPO in [C
N]). The selective synergistic effect for heavier Ln(III) ions was found using a combination of Htta and TOPO in [C mim][Tf
mim][Tf N], leading to enhanced separability among Ln(III) ions. The extracted Ln(III) species and the extraction constants in the Htta-TOPO system were determined by three-dimensional extraction equilibrium analysis. The selective synergism for heavier Ln(III) ions in the Htta-TOPO system was ascribed to the formation of hydrophobic, charged adducts, such as Ln(tta)
N], leading to enhanced separability among Ln(III) ions. The extracted Ln(III) species and the extraction constants in the Htta-TOPO system were determined by three-dimensional extraction equilibrium analysis. The selective synergism for heavier Ln(III) ions in the Htta-TOPO system was ascribed to the formation of hydrophobic, charged adducts, such as Ln(tta) (TOPO)
(TOPO)
 and Ln(tta)(TOPO)
and Ln(tta)(TOPO)
 , in [C
, in [C mim][Tf
mim][Tf N].
N].
Shimojo, Kojiro
Analytical Sciences, 34(12), p.1345 - 1346, 2018/12
Solvent extraction is one of the most effective analytical methods for separation, purification, and removal of target metal ions (e.g. valuable metal ions, toxic metal ions and radioactive fission products) from aqueous solutions containing various metal ions. In this paper, we introduce the papers related to (1) novel extractant, (2) ionic liquids and deep eutectic solvents, (3) new extractors, among papers on solvent extraction published between 2016 and 2018.
Atanassova, M.*; Okamura, Hiroyuki; Eguchi, Ayano; Ueda, Yuki; Sugita, Tsuyoshi; Shimojo, Kojiro
Analytical Sciences, 34(8), p.973 - 978, 2018/08
Times Cited Count:18 Percentile:55.92(Chemistry, Analytical)The distribution constants of 4-benzoyl-3-phenyl-5-isoxazolone (HPBI) and deprotonated one (PBI ) between hydrophobic ionic liquid 1-butyl-3-methylimidazolium bis(trifluoromethanesulfonyl)imide ([C
) between hydrophobic ionic liquid 1-butyl-3-methylimidazolium bis(trifluoromethanesulfonyl)imide ([C C
C im][Tf
im][Tf N]) and aqueous phases were determined, together with the acid-dissociation constant of HPBI. The solvent extraction of three selected lanthanoid ions (La
N]) and aqueous phases were determined, together with the acid-dissociation constant of HPBI. The solvent extraction of three selected lanthanoid ions (La , Eu
, Eu , and Lu
, and Lu ) with HPBI from aqueous nitrate phase into [C
) with HPBI from aqueous nitrate phase into [C C
C im][Tf
im][Tf N] has been investigated. Application of the ionic liquid as the extracting phase greatly enhanced the extraction performance of HPBI for lanthanoid ions compared with that in the chloroform system. The composition of the extracted species was established to be anionic tetrakis entities, Ln(PBI)
N] has been investigated. Application of the ionic liquid as the extracting phase greatly enhanced the extraction performance of HPBI for lanthanoid ions compared with that in the chloroform system. The composition of the extracted species was established to be anionic tetrakis entities, Ln(PBI)
 , for light, middle, and heavy lanthanoid ions in an ionic environment.
, for light, middle, and heavy lanthanoid ions in an ionic environment.
Sugita, Tsuyoshi; Fujiwara, Iori*; Okamura, Hiroyuki; Oshima, Tatsuya*; Baba, Yoshinari*; Naganawa, Hirochika; Shimojo, Kojiro
Solvent Extraction Research and Development, Japan, 24(2), p.61 - 69, 2017/05
We investigated an influence of amide group in diglycolamic acid-type extractants on extraction property of metal ions. The extraction characteristics of  -dodecyldiglycolamic acid (C
-dodecyldiglycolamic acid (C DGAA), with a secondary amide group, for 56 metal ions have been investigated, and compared with those of
DGAA), with a secondary amide group, for 56 metal ions have been investigated, and compared with those of  -dioctyldiglycolamic acid (DODGAA) with a tertiary amide group. Compared with DODGAA, C
-dioctyldiglycolamic acid (DODGAA) with a tertiary amide group. Compared with DODGAA, C DGAA has a poor extraction performance and separation ability for rare-earth metal ions, except for Sc(III). However, C
DGAA has a poor extraction performance and separation ability for rare-earth metal ions, except for Sc(III). However, C DGAA tended to provide better extraction for relatively small-sized metal ions than DODGAA. In addition, it was found that C
DGAA tended to provide better extraction for relatively small-sized metal ions than DODGAA. In addition, it was found that C DGAA enables the selective removal of Hg(II) from aqueous solutions containing various divalent metal ions.
DGAA enables the selective removal of Hg(II) from aqueous solutions containing various divalent metal ions.
Shimojo, Kojiro
Nihon Ion Kokan Gakkai-Shi, 28(1), p.1 - 10, 2017/01
Development of metal separating reagents with high selectivity is necessary for efficient separation and recovery of valuable metals contained in industrial wastes, and removal and detection of toxic metals. We have developed novel metal separating ligands with a diglycolamic acid (DGAA) framework. The ligands have a tridentate coordination structure consisting of an amide group and a carboxy group connected by an ether chain and provide a metal separation ability superior to that of commercial ligands. Since the ligands can be synthesized simply in one step, this helps to reduce the production costs of the ligands. In the present review article, we report the extraction characteristics of DGAA-type ligand for 56 kinds of metal ions, extraction separation of rare-earth metal ions, and removal of toxic metal ions. Furthermore, a one-pot biological approach to fabricate DGAA-decorated gold nanoparticles has been developed using the DGAA-type ligand fused to the N-terminus of a gold-binding peptide. It was found that the DGAA-decorated gold nanoparticles can act as a high-sensitive colorimetric sensor for detecting toxic metal ions with color change.
 N
N anions in the ionic liquid-water distribution of europium(III) chelates
anions in the ionic liquid-water distribution of europium(III) chelatesOkamura, Hiroyuki; Aoyagi, Noboru; Shimojo, Kojiro; Naganawa, Hirochika; Imura, Hisanori*
RSC Advances (Internet), 7(13), p.7610 - 7618, 2017/01
Times Cited Count:20 Percentile:52.90(Chemistry, Multidisciplinary)The role of bis(trifluoromethanesulfonyl)imide (Tf N
N ) anions in the ionic liquid-water distribution systems of the Eu(III) chelates with 2-thenoyltrifluoroacetone (Htta) was investigated by the liquid-liquid distribution and time-resolved laser-induced fluorescence spectroscopy (TRLFS). The effect of the ionic liquids on the distribution constant of Eu(tta)
) anions in the ionic liquid-water distribution systems of the Eu(III) chelates with 2-thenoyltrifluoroacetone (Htta) was investigated by the liquid-liquid distribution and time-resolved laser-induced fluorescence spectroscopy (TRLFS). The effect of the ionic liquids on the distribution constant of Eu(tta) was evaluated by the regular solution theory. The distribution constant of Eu(tta)
was evaluated by the regular solution theory. The distribution constant of Eu(tta) in 1-alkyl-3-methylimidazolium bis(trifluoromethanesulfonyl)imide ([C
in 1-alkyl-3-methylimidazolium bis(trifluoromethanesulfonyl)imide ([C mim][Tf
mim][Tf N]) was increased dramatically by the solvation effects of Eu(tta)
N]) was increased dramatically by the solvation effects of Eu(tta) in [C
in [C mim][Tf
mim][Tf N]. TRLFS for [Eu(tta)
N]. TRLFS for [Eu(tta) (H
(H O)
O) ] synthesized revealed that the Eu(tta)
] synthesized revealed that the Eu(tta) chelate was almost completely dehydrated in a series of [C
chelate was almost completely dehydrated in a series of [C mim][Tf
mim][Tf N]. The Eu(tta)
N]. The Eu(tta) chelate exists as di- or tri-hydrates in 1-ethyl-3-methylimidazolium perchlorate ([C
chelate exists as di- or tri-hydrates in 1-ethyl-3-methylimidazolium perchlorate ([C mim][ClO
mim][ClO ]) containing 20 M water, whereas mono-hydrated chelate was formed in [C
]) containing 20 M water, whereas mono-hydrated chelate was formed in [C mim][Tf
mim][Tf N, ClO
N, ClO ] in the presence of 0.50 M Tf
] in the presence of 0.50 M Tf N
N and 20 M water. These results show that the coordinated water molecules of [Eu(tta)
and 20 M water. These results show that the coordinated water molecules of [Eu(tta) (H
(H O)
O) ] were replaced by the Tf
] were replaced by the Tf N
N anions. In fact, an anionic adduct, [Eu(tta)
anions. In fact, an anionic adduct, [Eu(tta) (Tf
(Tf N)]
N)] , was observed by electrospray ionization mass spectrometry in the presence of [C
, was observed by electrospray ionization mass spectrometry in the presence of [C mim][Tf
mim][Tf N].
N].
Okamura, Hiroyuki; Shimojo, Kojiro
Ion Ekitai Kenkyu Saizensen To Shakai Jisso, p.220 - 227, 2016/12
Solvent extraction is a separation method based on the difference in the distribution of solutes between two immiscible liquid phases. Recently, ionic liquids have been widely investigated as novel extraction media. Solvent properties of an ionic liquid can be adjusted by combination of a cationic and an anionic component. It is, therefore, possible to provide an attractive reaction field as an extraction medium. In this article, specific extraction phenomena observed in the ionic liquid extraction system for metal ions were introduced.
Shimojo, Kojiro; Fujiwara, Iori*; Fujisawa, Kiyoshi*; Okamura, Hiroyuki; Sugita, Tsuyoshi; Oshima, Tatsuya*; Baba, Yoshinari*; Naganawa, Hirochika
Solvent Extraction Research and Development, Japan, 23(2), p.151 - 159, 2016/05
Liquid-liquid extraction of rare-earth (RE) cations has been investigated using  -dodecyldiglycolamic acid (C
-dodecyldiglycolamic acid (C DGAA) with a secondary amide group, and compared with that using
DGAA) with a secondary amide group, and compared with that using  -dioctyldiglycolamic acid (DODGAA) with a tertiary amide group. C
-dioctyldiglycolamic acid (DODGAA) with a tertiary amide group. C DGAA enables quantitative transfer of all RE cations from moderately acidic solution, while being selective toward the heavier RE cations, and performs better than typical carboxylic-acid-type extractants. However, C
DGAA enables quantitative transfer of all RE cations from moderately acidic solution, while being selective toward the heavier RE cations, and performs better than typical carboxylic-acid-type extractants. However, C DGAA provides low extraction performance and separation ability for RE cations compared with DODGAA because of the weaker basicity of the amide oxygen. Slope analysis demonstrated that RE
DGAA provides low extraction performance and separation ability for RE cations compared with DODGAA because of the weaker basicity of the amide oxygen. Slope analysis demonstrated that RE transfer with C
transfer with C DGAA proceeded through a proton-exchange reaction, forming a 1:3 complex, RE(C
DGAA proceeded through a proton-exchange reaction, forming a 1:3 complex, RE(C DGAA)
DGAA) . Structural characterization by X-ray diffraction revealed that three
. Structural characterization by X-ray diffraction revealed that three  -butyldiglycolamic acid (C
-butyldiglycolamic acid (C DGAA) molecules coordinated to the La
DGAA) molecules coordinated to the La central ion in a tridentate fashion and the La
central ion in a tridentate fashion and the La primary coordination sphere consisted of three oxygen atoms from the amide group, three oxygen atoms from the ether group, and three oxygen atoms from the carboxy group.
primary coordination sphere consisted of three oxygen atoms from the amide group, three oxygen atoms from the ether group, and three oxygen atoms from the carboxy group.
 -bis(thiocyanato)aurate(I) complexes in ionic liquids
-bis(thiocyanato)aurate(I) complexes in ionic liquidsAoyagi, Noboru; Shinha, Yusuke*; Ikeda, Atsushi*; Haga, Yoshinori; Shimojo, Kojiro; Brooks, N. R.*; Izuoka, Akira*; Naganawa, Hirochika; Kimura, Takaumi; Binnemans, K.*
Crystal Growth & Design, 15(3), p.1422 - 1429, 2015/03
Times Cited Count:12 Percentile:64.39(Chemistry, Multidisciplinary)The photochemistry of a gold(I) thiocyanate complex has been investigated to determine the coordination structure in both the solid and liquid states. The coordination geometries of the supramolecular complex and the concomitant exciplex have mainly been analyzed by crystallographic analysis and X-ray absorption spectroscopy. The Au-S bond distance and Au-Au separation of the compound in the ground state and in the phosphorescent excited state were compared. Upon irradiation with UV light, the excimeric interactions were enhanced, resulting in a contraction of the Au-Au aurophilic distance. A broad luminescence spectrum was observed for the one-dimensional chain suprastructure. The time-resolved luminescence spectra indicated the entity of several oligomeric species in the crude liquid without neutral solvent molecules. In addition, EXAFS spectroscopy exhibited a slight change in the nearest Au-S distance due to the photo-switched transformation. The deformation of the (Au-Au)* exciplexes was not apparently promoted in the liquid state with the asymmetrical imidazoium cations having a non-local charge distribution in the present observation. This is plausibly due to a flexible molecular structure and a property in the liquid state. In conclusion, the photo-excited nature of the pseudo-one dimensional complexes is emerged in controlling bond distances among the supramolecular networks.
 -diketone fragments on the extraction of strontium into an ionic liquid
-diketone fragments on the extraction of strontium into an ionic liquidOkamura, Hiroyuki; Naganawa, Hirochika; Imura, Hisanori*; Shimojo, Kojiro
Proceedings of 20th International Solvent Extraction Conference (ISEC 2014), p.1046 - 1051, 2014/09
 -dioctyldiglycolamic acid DODGAA
-dioctyldiglycolamic acid DODGAAShimojo, Kojiro; Naganawa, Hirochika
Proceedings of 20th International Solvent Extraction Conference (ISEC 2014), p.1052 - 1057, 2014/09
Shimojo, Kojiro
Kagaku Kogaku, 78(7), P. 501, 2014/07
no abstracts in English
 ,
, -dioctyldiglycolamic acid
-dioctyldiglycolamic acidShimojo, Kojiro; Nakai, Ayaka*; Okamura, Hiroyuki; Saito, Takumi*; Ohashi, Akira*; Naganawa, Hirochika
Analytical Sciences, 30(4), p.513 - 517, 2014/04
Times Cited Count:18 Percentile:52.53(Chemistry, Analytical)