Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Hashimoto, Kei*; Shiwaku, Toru*; Aoki, Hiroyuki; Yokoyama, Hideaki*; Mayumi, Koichi*; Ito, Kozo*
Science Advances (Internet), 9(47), p.eadi8505_1 - eadi8505_8, 2023/11
Times Cited Count:1 Percentile:52.07(Multidisciplinary Sciences)Narita, Hirokazu*; Maeda, Motoki*; Tokoro, Chiharu*; Suzuki, Tomoya*; Tanaka, Mikiya*; Shiwaku, Hideaki; Yaita, Tsuyoshi
RSC Advances (Internet), 13(25), p.17001 - 17007, 2023/06
Times Cited Count:1 Percentile:51.1(Chemistry, Multidisciplinary)no abstracts in English
Shiwaku, Hideaki; Marushita, Motoharu*
JAEA-Research 2022-015, 39 Pages, 2023/05
We designed the hard X-ray undulator beamline BL22XU, which is dedicated to Japan Atomic Energy Research Institute (JAERI) at SPring-8 (now Japan Atomic Energy Agency (JAEA)). BL22XU is used for XAFS (X-ray Absorption Fine Structure) analysis experiments to develop separation and extraction materials for radioactive waste treatment and to elucidate their chemical behavior, magnetic research experiments using a diffractometer, and experiments under extreme conditions using a high-pressure press and a diamond anvil cell. The available X-ray energy range was set from 3 to 70 keV. To design the optics of the beamline, the reflectivity of the mirrors, the diffraction width of the monochromatic crystal, and the absorptance of the Be window were calculated. In addition, ray tracing was performed to optimize the materials for optics, dimensions, and location. The delay time of the ADL (Acoustic Delay Line) was also examined to ensure the safety in the use of radioactive materials. The operation of BL22XU "JAEA Actinide Science I" has already started. By collaborating BL22XU and BL23SU "JAEA Actinide Science II," which uses a soft X-ray undulator as a light source, we solve the problems to promote nuclear sciences. Since the monochromator was upgraded in 2018-2019, initial planning and measured data are documented here again.
Suzuki, Tomoya*; Otsubo, Ukyo*; Ogata, Takeshi*; Shiwaku, Hideaki; Kobayashi, Toru; Yaita, Tsuyoshi; Matsuoka, Mitsuaki*; Murayama, Norihiro*; Narita, Hirokazu*
Separation and Purification Technology, 308, p.122943_1 - 122943_7, 2023/03
Times Cited Count:2 Percentile:24.43(Engineering, Chemical)HNO leaching is used in recycling Pd metal from spent products that primarily contain Ag, and most Pd residues are separated from solutions containing Ag(I). However, a small amount of Pd(II) often remains in these Ag(I) solutions. Therefore, the separation of Pd(II) and Ag(I) in HNO
leaching is used in recycling Pd metal from spent products that primarily contain Ag, and most Pd residues are separated from solutions containing Ag(I). However, a small amount of Pd(II) often remains in these Ag(I) solutions. Therefore, the separation of Pd(II) and Ag(I) in HNO solutions is essential to promote efficient Pd recycling. In this study, the separation of Pd(II) and Ag(I) in HNO
solutions is essential to promote efficient Pd recycling. In this study, the separation of Pd(II) and Ag(I) in HNO solutions was investigated using four N-donor-type adsorbents functionalized with amine (R-Amine), iminodiacetic acid (R-IDA), pyridine (R-Py), or bis-picolylamine (R-BPA). R-Amine, R-IDA, and R-Py selectively adsorbed Pd(II) over Ag(I), Cu(II), Ni(II), and Fe(III) from HNO
solutions was investigated using four N-donor-type adsorbents functionalized with amine (R-Amine), iminodiacetic acid (R-IDA), pyridine (R-Py), or bis-picolylamine (R-BPA). R-Amine, R-IDA, and R-Py selectively adsorbed Pd(II) over Ag(I), Cu(II), Ni(II), and Fe(III) from HNO solutions (0.3-7 M), but R-Amine exhibited a lower Pd adsorption efficiency. In contrast,
solutions (0.3-7 M), but R-Amine exhibited a lower Pd adsorption efficiency. In contrast,  90% of Pd(II), Ag(I), and Cu(II) were adsorbed by R-BPA over the entire range of HNO
90% of Pd(II), Ag(I), and Cu(II) were adsorbed by R-BPA over the entire range of HNO concentrations. Structural analyses of the adsorbed metal ions using Fourier transform infrared spectroscopy and extended X-ray absorption fine structure spectroscopy revealed the separation mechanisms of the N-donor-type adsorbents. Pd(II) adsorption on R-IDA, R-Py, and R-BPA occurred via Pd(II) coordination of the functional groups (iminodiacetic acid, pyridine, and bis-picolylamine, respectively), whereas that on R-Amine occurred via anion exchange of NO
concentrations. Structural analyses of the adsorbed metal ions using Fourier transform infrared spectroscopy and extended X-ray absorption fine structure spectroscopy revealed the separation mechanisms of the N-donor-type adsorbents. Pd(II) adsorption on R-IDA, R-Py, and R-BPA occurred via Pd(II) coordination of the functional groups (iminodiacetic acid, pyridine, and bis-picolylamine, respectively), whereas that on R-Amine occurred via anion exchange of NO
 with [Pd(NO
with [Pd(NO )
) ]
] . The coordinative adsorption mechanisms resulted in the higher Pd(II) adsorption behaviors of R-IDA, R-Py, and R-BPA. HCl (5.0 M) and thiourea (0.1 M) eluents desorbed 83% of Pd(II) from R-IDA and 95% from R-Py, respectively. R-Py was the most effective Pd(II) adsorbent based on adsorption selectivity and desorption efficiency.
. The coordinative adsorption mechanisms resulted in the higher Pd(II) adsorption behaviors of R-IDA, R-Py, and R-BPA. HCl (5.0 M) and thiourea (0.1 M) eluents desorbed 83% of Pd(II) from R-IDA and 95% from R-Py, respectively. R-Py was the most effective Pd(II) adsorbent based on adsorption selectivity and desorption efficiency.
Okamoto, Yoshihiro; Shiwaku, Hideaki; Shimamura, Keisuke*; Kobayashi, Hidekazu; Nagai, Takayuki; Inose, Takehiko*; Sato, Seiichi*; Hatakeyama, Kiyoshi*
Journal of Nuclear Materials, 570, p.153962_1 - 153962_13, 2022/11
Simulated nuclear waste glass samples containing phosphorus, which increase the solubility of molybdenum, were prepared and analyzed using synchrotron X-ray Absorption Fine Structure (XAFS) analysis for some constituent elements and Raman spectroscopic analysis of their complex structure. Changes in local structure and chemical state due to different phosphorus additions and waste loading rates were systematically studied. Consequently, no crystalline phase due to the molybdate compound was observed even at a maximum waste content of 30 wt% (corresponding to 1.87 mol% MoO ). Oxidation proceeded when the waste-loading rate was increased, whereas the reduction proceeded when phosphorus was added. In some cases, the effects of oxidation and reduction were offset. The local structure around specific elements can be classified as follows; Zn that is affected mainly by the waste-loading rate, Ce that is affected by both the waste-loading rate and phosphorus addition, and Zr element that is not affected by either of them. From the comparison between the analytical results of Mo and other elements, it was considered that the added phosphorus exists as a free PO
). Oxidation proceeded when the waste-loading rate was increased, whereas the reduction proceeded when phosphorus was added. In some cases, the effects of oxidation and reduction were offset. The local structure around specific elements can be classified as follows; Zn that is affected mainly by the waste-loading rate, Ce that is affected by both the waste-loading rate and phosphorus addition, and Zr element that is not affected by either of them. From the comparison between the analytical results of Mo and other elements, it was considered that the added phosphorus exists as a free PO structural unit and may deprive the alkali metal coordinated to the molybdate ion.
structural unit and may deprive the alkali metal coordinated to the molybdate ion.
Takahatake, Yoko; Watanabe, So; Irisawa, Keita; Shiwaku, Hideaki; Watanabe, Masayuki
Journal of Nuclear Materials, 556, p.153170_1 - 153170_7, 2021/12
Times Cited Count:1 Percentile:16.35(Materials Science, Multidisciplinary)Okamoto, Yoshihiro; Osugi, Takeshi; Shiwaku, Hideaki; Akabori, Mitsuo*
Insights Concerning the Fukushima Daiichi Nuclear Accident, Vol.4; Endeavors by Scientists, p.285 - 294, 2021/10
The transfer behavior of cesium adsorbed on some clay minerals in aqueous solution was investigated by X-ray absorption fine structure (XAFS) analysis of the Cs K-edge. The sample was prepared by mixing Cs-adsorbed mineral with a different pure clay mineral in water. The XAFS results of the dried mixture powder were compared with those obtained before mixing. It was recognized from the XAFS analysis for three kinds of clay minerals illite, kaolinite and vermiculite, that cesium was transferred from kaolinite to illite and vermiculite, and from illite to vermiculite. It can be concluded that cesium is transferred to and accumulated in vermiculite.
Suzuki, Tomoya*; Otsubo, Ukyo*; Ogata, Takeshi*; Shiwaku, Hideaki; Kobayashi, Toru; Yaita, Tsuyoshi; Matsuoka, Mitsuaki*; Murayama, Norihiro*; Narita, Hirokazu*
Dalton Transactions (Internet), 50(33), p.11390 - 11397, 2021/09
Times Cited Count:2 Percentile:21.56(Chemistry, Inorganic & Nuclear)no abstracts in English
Okamoto, Yoshihiro; Kobayashi, Hidekazu; Shiwaku, Hideaki; Sasage, Kenichi; Hatakeyama, Kiyoshi*; Nagai, Takayuki
Journal of Non-Crystalline Solids, 551, p.120393_1 - 120393_8, 2021/01
Times Cited Count:5 Percentile:30.89(Materials Science, Ceramics)The chemical state of ruthenium in simulated iron phosphate radioactive waste glass was investigated by conventional X-ray absorption fine structure (XAFS) and imaging XAFS analyses. The EXAFS analysis suggested that ruthenium was contained as glass phase when content of the waste components was less than 10wt.% in 30 mol%Fe O
O -P
-P O
O base glass. In other samples, crystalline RuO
base glass. In other samples, crystalline RuO was predominant. According to the imaging XAFS analysis, RuO
was predominant. According to the imaging XAFS analysis, RuO particles in all samples had length smaller than 50
particles in all samples had length smaller than 50 m. Aggregations of RuO
m. Aggregations of RuO , which are found in nuclear waste borosilicate glass, were not seen in any of the iron phosphate glass samples.
, which are found in nuclear waste borosilicate glass, were not seen in any of the iron phosphate glass samples.
Sugiura, Yuki; Tomura, Tsutomu*; Ishidera, Takamitsu; Doi, Reisuke; Francisco, P. C. M.; Shiwaku, Hideaki; Kobayashi, Toru; Matsumura, Daiju; Takahashi, Yoshio*; Tachi, Yukio
Journal of Radioanalytical and Nuclear Chemistry, 324(2), p.615 - 622, 2020/05
Times Cited Count:4 Percentile:45.45(Chemistry, Analytical)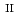 and Si under anoxic and reducing conditions; Structural characteristics of ferrous silicate co-precipitates
and Si under anoxic and reducing conditions; Structural characteristics of ferrous silicate co-precipitatesFrancisco, P. C. M.; Mitsui, Seiichiro; Ishidera, Takamitsu; Tachi, Yukio; Doi, Reisuke; Shiwaku, Hideaki
Geochimica et Cosmochimica Acta, 270, p.1 - 20, 2020/02
Times Cited Count:15 Percentile:76.71(Geochemistry & Geophysics)Suzuki, Tomoya*; Ogata, Takeshi*; Tanaka, Mikiya*; Kobayashi, Toru; Shiwaku, Hideaki; Yaita, Tsuyoshi; Narita, Hirokazu*
Analytical Sciences, 35(12), p.1353 - 1360, 2019/12
Times Cited Count:3 Percentile:12.45(Chemistry, Analytical)no abstracts in English
Yoneda, Yasuhiro; Yoshigoe, Akitaka; Takeda, Yukiharu; Shiwaku, Hideaki; Matsumura, Daiju; Shobu, Takahisa; Tamura, Kazuhisa
Materia, 58(12), p.763 - 769, 2019/12
This is an introduction to the equipment provided for each implementation period belonging to the structure analysis platform in the nanotechnology platform.
Nagai, Takayuki; Sasage, Kenichi; Okamoto, Yoshihiro; Shiwaku, Hideaki; Yamagishi, Hirona*; Ota, Toshiaki*; Inose, Takehiko*; Sato, Seiichi*; Hatakeyama, Kiyoshi*; Takahashi, Tomoe*; et al.
JAEA-Research 2019-003, 94 Pages, 2019/09
The local structures of glass-forming elements and waste elements would change by the chemical composition of waste glass including those elements. In this study, simulated waste glass samples were prepared from borosilicate glass frit including phosphorus (P) or vanadium (V), and we investigated local structures of boron, sodium, and waste elements in these P glass and V glass samples by using synchrotron XAFS measurements in soft and hard X ray region.
Narita, Hirokazu*; Nicolson, R. M.*; Motokawa, Ryuhei; Ito, Fumiyuki*; Morisaku, Kazuko*; Goto, Midori*; Tanaka, Mikiya*; Heller, W. T.*; Shiwaku, Hideaki; Yaita, Tsuyoshi; et al.
Inorganic Chemistry, 58(13), p.8720 - 8734, 2019/07
Times Cited Count:14 Percentile:69.69(Chemistry, Inorganic & Nuclear) -trimethylglycine
-trimethylglycineSuzuki, Tomoya*; Narita, Hirokazu*; Ogata, Takeshi*; Suzuki, Hideya; Matsumura, Tatsuro; Kobayashi, Toru; Shiwaku, Hideaki; Yaita, Tsuyoshi
Solvent Extraction Research and Development, Japan, 26(1), p.11 - 19, 2019/06
Times Cited Count:3 Percentile:14.02(Chemistry, Multidisciplinary)The ability of AMP03, a styrene-divinylbenzene copolymer functionalized with  -trimethylglycine moieties, to adsorb Pd(II) from HNO
-trimethylglycine moieties, to adsorb Pd(II) from HNO solutions was investigated to elucidate the affinity of
solutions was investigated to elucidate the affinity of  -trimethylglycine for Pd(II). In the present study, we investigated the mechanism of Pd(II) adsorption by AMP03 by means of adsorption experiments, Fourier Transform Infrared (FT-IR) spectroscopy, and Extended X-ray Absorption Fine Structure (EXAFS) spectroscopy.
-trimethylglycine for Pd(II). In the present study, we investigated the mechanism of Pd(II) adsorption by AMP03 by means of adsorption experiments, Fourier Transform Infrared (FT-IR) spectroscopy, and Extended X-ray Absorption Fine Structure (EXAFS) spectroscopy.
Okamoto, Yoshihiro; Nagai, Takayuki; Shiwaku, Hideaki; Kano, Shigeru; Himeno, Haruyuki*; Kobayashi, Hiroshi*; Nakatani, Mikio*
JAEA-Research 2018-013, 18 Pages, 2019/03
The chemical state and local structure of some elements in the simulated nuclear waste glass samples (20 batches) prepared by bottom drain test in the full scale mock-up tests using KMOC melter were investigated by synchrotron radiation based X-ray absorption fine structure (XAFS) analysis. As a result of the analysis of cerium element, it was confirmed that the oxidation proceeds gently as the batch advanced. For manganese, iron, and zinc, there was almost no difference between batches, which seemed to be stabilized by getting into the frame structure of the borosilicate glass. There were no elements that seemed to be clearly crystalline except for platinum group elements. Remarkable precipitation was hardly observed in zirconium and molybdenum with the imaging analysis.
Ambai, Hiromu; Nishizuka, Yusuke*; Sano, Yuichi; Uchida, Naoki; Iijima, Shizuka; Shiwaku, Hideaki; Yaita, Tsuyoshi
Journal of Nuclear Science and Technology, 56(2), p.193 - 200, 2019/02
Times Cited Count:1 Percentile:11.15(Nuclear Science & Technology)During the accident that occurred at the Fukushima Daiichi Nuclear Power Plant, a large volume of seawater was introduced as coolant into the storage pools for spent nuclear fuel. If this fuel is reprocessed, some components of seawater will be mixed with the nitric acid solution containing metal ions in the reprocessing process where stainless steels are used as structural material. In this study, we investigated the effect of seawater components in high active liquid waste (HAW) containing nitric acid and metal ions as fission products on the corrosion behavior of SUS316L stainless steel. Corrosion tests were conducted in surrogate HAW containing artificial seawater (ASW). Intergranular corrosion was observed in the HAW with ASW, where Ru increased the corrosion potential to the transpassive region. An increase in the amount of ASW led to a decrease in the corrosion rate and suppression of intergranular corrosion. Interactions between Ru ions and seawater components, such as chloride ions, were indicated by the results of extended Xray absorption fine structure spectroscopy and cyclic voltammetry analyses of the solution containing ASW and HAW.
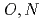 -hetero donor ligand BIZA
-hetero donor ligand BIZAKobayashi, Toru; Akutsu, Kazuhiro*; Nakase, Masahiko*; Suzuki, Shinichi; Shiwaku, Hideaki; Yaita, Tsuyoshi
Separation Science and Technology, 54(13), p.2077 - 2083, 2019/02
Times Cited Count:5 Percentile:22.52(Chemistry, Multidisciplinary)Development of separation reagent, which can efficiently separate lanthanides, is of increasing importance because it concerns establishment of purification and recycle techniques rare-earth elements. In our research group, several hetero donor type ligands, which have both oxygen and heterocyclic nitrogen atoms as donor atoms, were developed and revealed that this type of ligands show the selective complexation with specific lanthanide. In this paper, we will discuss the lanthaides complexation properties of the hetero donor type ligand containing benzimidazole group as nitrogen donor ( -methyl-
-methyl- -phenyl-2-(1
-phenyl-2-(1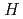 -benzimidazol-2-yl)-pyridine-6-carboxamide) based on structural investigation by crystallography.
-benzimidazol-2-yl)-pyridine-6-carboxamide) based on structural investigation by crystallography.
Okamoto, Yoshihiro; Nagai, Takayuki; Shiwaku, Hideaki
Yoyuen Oyobi Koon Kogaku, 62(1), p.11 - 17, 2019/01
The vitrified radioactive waste is a multicomponent material containing many kinds of elements. Synchrotron radiation XAFS with element selectivity is suitable for analysis of elements in the waste. From the XAFS analysis, the chemical state and the local structure of each element were clarified. Imaging XAFS technique was used as an analysis based on element distribution in the glass. The imaging XAFS is effective for analysis of elements that are less soluble in the glass like molybdenum and platinum group elements. It was clarified from the simultaneous imaging XAFS analyses of multiple elements that the chemical form of rhodium is strongly dominated by the distribution correlation with ruthenium. We proposed multi - scale structural analyses with wide angle scattering, PDF analysis, small angle scattering in order to evaluate the soundness of the vitrified waste.