Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Abe, Mitsushi*; Bae, S.*; Beer, G.*; Bunce, G.*; Choi, H.*; Choi, S.*; Chung, M.*; da Silva, W.*; Eidelman, S.*; Finger, M.*; et al.
Progress of Theoretical and Experimental Physics (Internet), 2019(5), p.053C02_1 - 053C02_22, 2019/05
Times Cited Count:161 Percentile:99.30(Physics, Multidisciplinary)This paper introduces a new approach to measure the muon magnetic moment anomaly 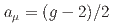 and the muon electric dipole moment (EDM)
and the muon electric dipole moment (EDM) 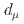 at the J-PARC muon facility. The goal of our experiment is to measure
at the J-PARC muon facility. The goal of our experiment is to measure 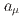 and
and 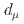 using an independent method with a factor of 10 lower muon momentum, and a factor of 20 smaller diameter storage-ring solenoid compared with previous and ongoing muon g-2 experiments with unprecedented quality of the storage magnetic field. Additional significant differences from the present experimental method include a factor of 1000 smaller transverse emittance of the muon beam (reaccelerated thermal muon beam), its efficient vertical injection into the solenoid, and tracking each decay positron from muon decay to obtain its momentum vector. The precision goal for
using an independent method with a factor of 10 lower muon momentum, and a factor of 20 smaller diameter storage-ring solenoid compared with previous and ongoing muon g-2 experiments with unprecedented quality of the storage magnetic field. Additional significant differences from the present experimental method include a factor of 1000 smaller transverse emittance of the muon beam (reaccelerated thermal muon beam), its efficient vertical injection into the solenoid, and tracking each decay positron from muon decay to obtain its momentum vector. The precision goal for 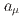 is a statistical uncertainty of 450 parts per billion (ppb), similar to the present experimental uncertainty, and a systematic uncertainty less than 70 ppb. The goal for EDM is a sensitivity of
is a statistical uncertainty of 450 parts per billion (ppb), similar to the present experimental uncertainty, and a systematic uncertainty less than 70 ppb. The goal for EDM is a sensitivity of 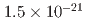 e
e cm.
cm.
Mukai, Yasunobu; Shoji, Kaoru; Hayashi, Hiroyuki*; Nakamura, Hironobu; Kurita, Tsutomu
Proceedings of INMM 53rd Annual Meeting (CD-ROM), 9 Pages, 2012/07
In general, electro manometer system for volume measurement in high concentrated Pu, U solution is used. The measurement uncertainty of volume measurement should be kept within 0.36% in the nuclear material accountancy. Not only manometer censors are calibrated periodically, but also operator should maintain the integrity of entire volume measurement system. If clogging at a tip of dip-tube is occurred, it causes measurement bias. Therefore, we studied confirmation procedure of healthiness in the system during solution storage. As result, it is confirmed that following points are effective: (1) Confirmation of consistency in the Kumar's density formula by using actual Pu, U concentration, density and acidity determined by periodical sampling and high accuracy DA. In addition, Pu, U mass is maintained within the uncertainty from the previous sampling/DA result. (2) In order to confirm the integrity of differential pressure for density that may be impacted regarding the measurement bias by clogging, comparison between measured differential pressure for density and analyzed density with high accuracy is important. In the confirmation for density, correction method between different temperatures was established by the result of evaluation of temperature effect derived by Kumar's density formula.
Iijima, Kazuki; Tomura, Tsutomu*; Shoji, Yoshiyuki*
Applied Clay Science, 49(3), p.262 - 268, 2010/06
Times Cited Count:43 Percentile:73.27(Chemistry, Physical)Sorption and desorption behavior of Cs onto montmorillonite colloids was investigated. The ion exchange and surface complexation model can successfully reproduce the sorption and desorption behavior of Cs. Conditioning montmorillonite in higher Cs concentration than 0.005M causes decrease of interlayer distance which may lead to fixation of Cs in the interlayer. Sorption reversibility of Cs on montmorillonite colloids can be expected in this study due to lower Cs concentration than 0.0001M.
Iijima, Kazuki; Shoji, Yoshiyuki*; Tomura, Tsutomu*
Radiochimica Acta, 96(9-11), p.721 - 730, 2008/00
Times Cited Count:14 Percentile:65.33(Chemistry, Inorganic & Nuclear)Distribution coefficients of Am onto bentonite colloid were evaluated under weak basic and low ionic strength conditions, in taking the influence of Am colloid into account. The obtained values are larger than those obtained for larger montmorillonite particles in literatures. It is attributed to one order of magnitude higher reactive site density of the bentonite colloid. Applicability of a relatively simple and generalized mechanistic sorption model to the bentonite colloid is also discussed.
Tomita, Yutaka; Morihira, Masayuki; Tamaki, Yoshihisa*; Nishimura, Kazuhisa*; Shoji, Shuichi*; Kihara, Yoshiyuki; Kase, Takeshi; Koizumi, Tsutomu
JAEA-Research 2006-088, 95 Pages, 2007/01
JAEA has developed sphere-pac fuels in the feasibility study on commercialized FBR cycle systems as one of the candidates for low decontamination TRU fuels. Optimization of the fabrication condition for coarse spheres, development of an improved external gelation process, and examination of peculiar problems for the low decontamination fuel were carried out in Phase II. The results are shown as follows. (1) Fabrication condition of coarse spheres was optimized. (2) Feasibility of the improved external gelation process was confirmed. (3) Rare earth elements did not bring any problem for the characteristic of spheres and fabrication condition. (4) Radiation resistant data of the feed solution was acquired. Results of tests show the feasibility of the external gelation process for the low decontamination TRU fuel microsphere fabrication.
Shimomura, Yasuo; Tsunematsu, Toshihide; Yamamoto, Shin; Maruyama, So; Mizoguchi, Tadanori*; Takahashi, Yoshikazu; Yoshida, Kiyoshi; Kitamura, Kazunori*; Ioki, Kimihiro*; Inoue, Takashi; et al.
Purazuma, Kaku Yugo Gakkai-Shi, 78(Suppl.), 224 Pages, 2002/01
no abstracts in English
Kambara, Toyozo; Uno, Hidero; Shoda, Katsuhiko; Hirata, Yutaka; Shoji, Tsutomu; Kohayakawa, Toru; Takayanagi, Hiroshi; Fujimura, Tsutomu; Morita, Morito; Ichihara, Masahiro; et al.
JAERI 1045, 11 Pages, 1963/03
no abstracts in English
JRR-2 Control Office; Kambara, Toyozo; Shoda, Katsuhiko; Hirata, Yutaka; Shoji, Tsutomu; Kohayakawa, Toru; Morozumi, Minoru; Kambayashi, Yuichiro; Shitomi, Hajimu; Kokanezawa, Takashi; et al.
JAERI 1027, 57 Pages, 1962/09
no abstracts in English
Kambara, Toyozo; Shoda, Katsuhiko; Hirata, Yutaka; Shoji, Tsutomu; Haginoya, Kinichi; Kohayakawa, Toru; Yamaki, Jikei; Yokota, Mitsuo; Horiki, Oichiro; Yuhara, Shunichi; et al.
JAERI 1023, 120 Pages, 1962/09
no abstracts in English
JRR-2 Operations Office; Kambara, Toyozo; Shoda, Katsuhiko; Hirata, Yutaka; Shoji, Tsutomu; Haginoya, Kinichi; Kohayakawa, Toru; Yamaki, Jikei; Yokota, Mitsuo; Horiki, Oichiro; et al.
JAERI 1024, 79 Pages, 1962/08
no abstracts in English
JRR-2 Critical Experiments Group; Kambara, Toyozo; Shoda, Katsuhiko; Hirata, Yutaka; Shoji, Tsutomu; Kohayakawa, Toru; Morozumi, Minoru; Kambayashi, Yuichiro; Shitomi, Hajimu; Kokanezawa, Takashi; et al.
JAERI 1025, 62 Pages, 1962/03
no abstracts in English
Iijima, Kazuki; Shoji, Yoshiyuki; Tomura, Tsutomu*
no journal, ,
Sorption site density of bentonite was investigated by acid titration. As a result, bentonite colloid is considered to have higher sorption site density than that of larger bentonite particle. Thus the high sorption site density, which has been supposed to lead to the large distribution coefficient of Am onto the bentonite colloid in the previous presentation, is experimentally proved.
Iijima, Kazuki; Shoji, Yoshiyuki; Tomura, Tsutomu*
no journal, ,
Sorption behavior of Am onto bentonite colloid was investigated. The obtained distribution coefficients were larger than those evaluated by the calculation in which ion exchange and surface complexation reactions were assumed. It seems to be due to the higher site density of bentonite colloid for surface complexation compared with bulk bentonite.
Iijima, Kazuki; Shoji, Yoshiyuki; Tomura, Tsutomu*
no journal, ,
Sorption behavior of Am onto bentonite colloid was investigated. Distribution coefficient of Am was evaluated taking account for the existence of Am colloid. Obtained values were larger than those obtained with larger bentonite particles included in the JNC-SDB. In the acid-base titration, bentonite colloid shows larger edge site density than larger bentonite particles. In the calculation by sorption model assuming ion exchange and surface complexation, similar distribution coefficient was obtained at pH 8 with this edge site density. Based on these results, it is considered that larger distribution coefficient of bentonite colloid is due to the higher edge site density, which is dominant for the Am sorption.