Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Khuyagbaatar, J.*; Yakushev, A.*; D llmann, Ch. E.*; Ackermann, D.*; Andersson, L.-L.*; Asai, Masato; Block, M.*; Boll, R. A.*; Brand, H.*; Cox, D. M.*; et al.
llmann, Ch. E.*; Ackermann, D.*; Andersson, L.-L.*; Asai, Masato; Block, M.*; Boll, R. A.*; Brand, H.*; Cox, D. M.*; et al.
Physical Review C, 102(6), p.064602_1 - 064602_9, 2020/12
Times Cited Count:78 Percentile:99.01(Physics, Nuclear)A search for production of the superheavy elements with atomic numbers 119 and 120 was performed in the  Ti+
Ti+ Bk and
Bk and  Ti+
Ti+ Cf fusion-evaporation reactions, respectively, at the gas-filled recoil separator TASCA. Over four months of irradiation, neither was detected at cross-section sensitivity levels of 65 and 200 fb, respectively. The non-observation of elements 119 and 120 is discussed within the concept of fusion-evaporation reactions including various theoretical predictions on the fission-barrier heights of superheavy nuclei in the region of the island of stability.
Cf fusion-evaporation reactions, respectively, at the gas-filled recoil separator TASCA. Over four months of irradiation, neither was detected at cross-section sensitivity levels of 65 and 200 fb, respectively. The non-observation of elements 119 and 120 is discussed within the concept of fusion-evaporation reactions including various theoretical predictions on the fission-barrier heights of superheavy nuclei in the region of the island of stability.
 Ca+
Ca+ Bk leading to formation of the element Ts (Z=117)
Bk leading to formation of the element Ts (Z=117)Khuyagbaatar, J.*; Yakushev, A.*; D llmann, Ch. E.*; Ackermann, D.*; Andersson, L.-L.*; Asai, Masato; Block, M.*; Boll, R. A.*; Brand, H.*; Cox, D. M.*; et al.
llmann, Ch. E.*; Ackermann, D.*; Andersson, L.-L.*; Asai, Masato; Block, M.*; Boll, R. A.*; Brand, H.*; Cox, D. M.*; et al.
Physical Review C, 99(5), p.054306_1 - 054306_16, 2019/05
Times Cited Count:33 Percentile:92.64(Physics, Nuclear)We have performed an experiment to synthesize the element 117 (Ts) with the  Ca+
Ca+ Bk fusion reaction. Four
Bk fusion reaction. Four  -decay chains attributed to the element 117 were observed. Two of them were long decay chains which can be assigned to the one originating from the
-decay chains attributed to the element 117 were observed. Two of them were long decay chains which can be assigned to the one originating from the  decay of
decay of 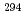 Ts. The other two were short decay chains which are consistent with the one originating from the
Ts. The other two were short decay chains which are consistent with the one originating from the  decay of
decay of 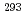 Ts. We have compared the present results with the literature data, and found that our present results mostly confirmed the literature data, leading to the firm confirmation of the synthesis of the element 117.
Ts. We have compared the present results with the literature data, and found that our present results mostly confirmed the literature data, leading to the firm confirmation of the synthesis of the element 117.
Eichler, R.*; Asai, Masato; Brand, H.*; Chiera, N. M.*; Di Nitto, A.*; Dressler, R.*; D llmann, Ch. E.*; Even, J.*; Fangli, F.*; Goetz, M.*; et al.
llmann, Ch. E.*; Even, J.*; Fangli, F.*; Goetz, M.*; et al.
EPJ Web of Conferences, 131, p.07005_1 - 07005_7, 2016/12
Times Cited Count:3 Percentile:71.15(Chemistry, Inorganic & Nuclear)In recent years gas-phase chemical studies assisted by physical pre-separation allowed for the productions and investigations of fragile single molecular species of superheavy elements. The latest highlight is the formation of very volatile hexacarbonyl compound of element 106, Sg(CO) . Following this success, second-generation experiments were performed to measure the first bond dissociation energy between the central metal atom and the surrounding ligand. The method using a tubular decomposition reactor was developed and successfully applied to short-lived Mo(CO)
. Following this success, second-generation experiments were performed to measure the first bond dissociation energy between the central metal atom and the surrounding ligand. The method using a tubular decomposition reactor was developed and successfully applied to short-lived Mo(CO) , W(CO)
, W(CO) , and Sg(CO)
, and Sg(CO) .
.
 at the single-atom level
at the single-atom levelSteinegger, P.*; Asai, Masato; Dressler, R.*; Eichler, R.*; Kaneya, Yusuke*; Mitsukai, Akina*; Nagame, Yuichiro; Piguet, D.*; Sato, Tetsuya; Sch del, M.; et al.
del, M.; et al.
Journal of Physical Chemistry C, 120(13), p.7122 - 7132, 2016/04
Times Cited Count:28 Percentile:63.01(Chemistry, Physical)A new experimental method "vacuum chromatography" has been developed to measure adsorption enthalpy of superheavy elements, and its feasibility has been examined using short-lived thallium isotopes. The short-lived thallium isotopes were produced at the JAEA tandem accelerator. The thallium ion beam prepared with an on-line isotope separator which ionized and mass-separated the thallium isotopes was injected into an isothermal vacuum chromatography apparatus. A temperature-dependent adsorption property of thallium atom on SiO surface were measured. The adsorption enthalpy of thallium was determined to be 158 kJ/mol. The thallium is a homolog of element 113. Thus, the vacuum chromatography developed in this study enables us to perform chemical experiments for short-lived superheavy elements with half-lives of a order of one second.
surface were measured. The adsorption enthalpy of thallium was determined to be 158 kJ/mol. The thallium is a homolog of element 113. Thus, the vacuum chromatography developed in this study enables us to perform chemical experiments for short-lived superheavy elements with half-lives of a order of one second.
 and W(CO)
and W(CO)
Usoltsev, I.*; Eichler, R.*; Wang, Y.*; Even, J.*; Yakushev, A.*; Haba, Hiromitsu*; Asai, Masato; Brand, H.*; Di Nitto, A.*; D llmann, Ch. E.*; et al.
llmann, Ch. E.*; et al.
Radiochimica Acta, 104(3), p.141 - 151, 2016/03
Times Cited Count:34 Percentile:94.30(Chemistry, Inorganic & Nuclear)Conditions of the production and decomposition of hexacarbonyl complexes of short-lived Mo and W isotopes were investigated to study thermal stability of the heaviest group 6 hexacarbonyl complex Sg(CO) . A tubular flow reactor was tested to decompose the hexacarbonyl complexes and to extract the first bond dissociation energies. A silver was found to be the most appropriate reaction surface to study the decomposition of the group 6 hexacarbonyl. It was found that the surface temperature at which the decomposition occurred was correlated to the first bond dissociation energy of Mo(CO)
. A tubular flow reactor was tested to decompose the hexacarbonyl complexes and to extract the first bond dissociation energies. A silver was found to be the most appropriate reaction surface to study the decomposition of the group 6 hexacarbonyl. It was found that the surface temperature at which the decomposition occurred was correlated to the first bond dissociation energy of Mo(CO) and W(CO)
and W(CO) , indicating that the first bond dissociation energy of Sg(CO)
, indicating that the first bond dissociation energy of Sg(CO) could be determined with this technique.
could be determined with this technique.
 Ca +
Ca +  Bk fusion reaction leading to element Z = 117; Long-lived
Bk fusion reaction leading to element Z = 117; Long-lived  -decaying
-decaying 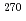 Db and discovery of
Db and discovery of 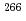 Lr
LrKhuyagbaatar, J.*; Yakushev, A.*; D llmann, Ch. E.*; Ackermann, D.*; Andersson, L.-L.*; Asai, Masato; Block, M.*; Boll, R. A.*; Brand, H.*; Cox, D. M.*; et al.
llmann, Ch. E.*; Ackermann, D.*; Andersson, L.-L.*; Asai, Masato; Block, M.*; Boll, R. A.*; Brand, H.*; Cox, D. M.*; et al.
Physical Review Letters, 112(17), p.172501_1 - 172501_5, 2014/05
Times Cited Count:232 Percentile:98.35(Physics, Multidisciplinary)The superheavy element with atomic number 117 was produced in the  Ca +
Ca +  Bk fusion reaction using the gas-filled recoil separator TASCA at GSI in Germany. This result verified the previous result of the discovery of new element 117 reported by Flerov Laboratory of Nuclear Reactions in Russia, which makes certain the synthesis and discovery of element 117 in human history. On the other hand, the last nucleus in the
Bk fusion reaction using the gas-filled recoil separator TASCA at GSI in Germany. This result verified the previous result of the discovery of new element 117 reported by Flerov Laboratory of Nuclear Reactions in Russia, which makes certain the synthesis and discovery of element 117 in human history. On the other hand, the last nucleus in the  decay chain from the element 117 was assigned to be the unknown nucleus
decay chain from the element 117 was assigned to be the unknown nucleus 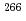 Lr instead of the previously reported
Lr instead of the previously reported 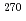 Db, and
Db, and 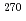 Db was found to be the
Db was found to be the  -decaying nucleus with very long half-life.
-decaying nucleus with very long half-life.
Kaneya, Yusuke*; Asai, Masato; Sato, Tetsuya; Tomitsuka, Tomohiro; Tsukada, Kazuaki; Toyoshima, Atsushi; Mitsukai, Akina; Makii, Hiroyuki; Hirose, Kentaro; Osa, Akihiko; et al.
no journal, ,
To study the influence of the valence 7p electronic orbital on chemical properties of lawrencium, a measurement of the adsorption enthalpy of lawrencium was carried out. A new method using a surface ionization technique coupled to an on-line isotope separator was developed, which enabled one to measure temperature dependence of lawrencium surface adsorption on a metallic tantalum surface at high temperature up to 2800 K. The temperature dependences of adsorption of lawrencium as well as various lanthanide elements were investigated with this method, and the adsorption enthalpy of lawrencium was successfully extracted.
electronic orbital on chemical properties of lawrencium, a measurement of the adsorption enthalpy of lawrencium was carried out. A new method using a surface ionization technique coupled to an on-line isotope separator was developed, which enabled one to measure temperature dependence of lawrencium surface adsorption on a metallic tantalum surface at high temperature up to 2800 K. The temperature dependences of adsorption of lawrencium as well as various lanthanide elements were investigated with this method, and the adsorption enthalpy of lawrencium was successfully extracted.
Sato, Tetsuya; Kaneya, Yusuke*; Asai, Masato; Tsukada, Kazuaki; Toyoshima, Atsushi; Mitsukai, Akina*; Osa, Akihiko; Makii, Hiroyuki; Nishio, Katsuhisa; Hirose, Kentaro; et al.
no journal, ,
The ground state electronic configuration of lawrencium (Lr, Z =103) is predicted to be [Rn]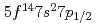 , which is different from that of the lanthanide homolog Lu [Xe]
, which is different from that of the lanthanide homolog Lu [Xe]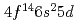 due to strong relativistic effects. According to semi-empirical considerations, volatility of Lr is expected to be higher than that of Lu. We have investigated adsorption behavior of
due to strong relativistic effects. According to semi-empirical considerations, volatility of Lr is expected to be higher than that of Lu. We have investigated adsorption behavior of  Lr, which was produced in the reaction of
Lr, which was produced in the reaction of  Cf(
Cf(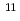 B, 4n), on a tantalum (Ta) metal surface using a surface ion-source installed into the isotope separator on-line (ISOL) at the JAEA tandem accelerator facility. The observed adsorption behavior of
B, 4n), on a tantalum (Ta) metal surface using a surface ion-source installed into the isotope separator on-line (ISOL) at the JAEA tandem accelerator facility. The observed adsorption behavior of  Lr was similar to those of Tb and Lu which have relatively higher adsorption enthalpy on Ta surface. It implies that Lr would have low volatility like such as Lu and Tb.
Lr was similar to those of Tb and Lu which have relatively higher adsorption enthalpy on Ta surface. It implies that Lr would have low volatility like such as Lu and Tb.
Kaneya, Yusuke*; Tomitsuka, Tomohiro; Sato, Tetsuya; Asai, Masato; Tsukada, Kazuaki; Toyoshima, Atsushi; Mitsukai, Akina; Makii, Hiroyuki; Hirose, Kentaro; Osa, Akihiko; et al.
no journal, ,