Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Sudo, Ayako; M sz
sz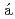 ros, B.*; Sato, Takumi; Nagae, Yuji
ros, B.*; Sato, Takumi; Nagae, Yuji
JAEA-Research 2023-007, 31 Pages, 2023/11
For the criticality assessment of fuel debris generated by the accident in Fukushima Daiichi Nuclear Power Station, understanding of the elemental localization in fuel debris is important. Especially, the distribution of Fe and Gd, which may behave as potential neutron absorber materials in the fuel debris, is of particular important from the viewpoint of nuclear criticality safety. To investigate the localization tendency of Gd and Fe in molten core materials during solidification progress, liquefaction/solidification tests on core materials containing UO , ZrO
, ZrO , FeO, Gd
, FeO, Gd O
O , and simulated fission products (MoO
, and simulated fission products (MoO , Nd
, Nd O
O , SrO, and RuO
, SrO, and RuO ) and concrete (SiO
) and concrete (SiO , Al
, Al O
O , and CaO) were performed using cold crucible induction heating technique. During the test, the molten core materials gradually subsided and solidified from the bottom to the top of the melt. Elemental analysis showed that Fe content in the inner region increased approximately up to 3.4 times that in the bottom region. The concentration of Fe into the inner region was observed in all the samples regardless of the initial FeO composition, cooling rates, and phase separation. This suggests that FeO may be concentrated into the low temperature region, where the melt solidified later. In contrast, Gd content in the bottom region increased approximately up to 2.6 times that in the inner region. The concentration of Gd into the bottom region was observed when the initial Gd
, and CaO) were performed using cold crucible induction heating technique. During the test, the molten core materials gradually subsided and solidified from the bottom to the top of the melt. Elemental analysis showed that Fe content in the inner region increased approximately up to 3.4 times that in the bottom region. The concentration of Fe into the inner region was observed in all the samples regardless of the initial FeO composition, cooling rates, and phase separation. This suggests that FeO may be concentrated into the low temperature region, where the melt solidified later. In contrast, Gd content in the bottom region increased approximately up to 2.6 times that in the inner region. The concentration of Gd into the bottom region was observed when the initial Gd O
O content was higher than 1 at.%. This suggests that Gd
content was higher than 1 at.%. This suggests that Gd O
O may be concentrated into the earlier solidified region. On the other hand, no significant localization was observed on the simulated fission products.
may be concentrated into the earlier solidified region. On the other hand, no significant localization was observed on the simulated fission products.
 temperature measurement for lamp-based heating device
temperature measurement for lamp-based heating deviceSumita, Takehiro; Sudo, Ayako; Takano, Masahide; Ikeda, Atsushi
Science and Technology of Advanced Materials; Methods (Internet), 2(1), p.50 - 54, 2022/02
Sudo, Ayako; Sato, Takumi; Ogi, Hiroshi; Takano, Masahide
Journal of Nuclear Science and Technology, 58(4), p.473 - 481, 2021/04
Times Cited Count:5 Percentile:65.59(Nuclear Science & Technology)Dissolution behavior of Sr and Ba is crucial for evaluating secondary source terms via coolant water from ex-vessel debris accumulated at Fukushima Daiichi Nuclear Power Plant. To understand the mechanism, knowing the distribution of Sr and Ba in the ex-vessel debris is necessary. As a result of reaction tests between simulated corium and concrete materials, two layered structures were observed in the solidified sample, (A) a silicate glass-based ((Si-Al-Ca-Fe-Zr-Cr-U-Sr-Ba)-O) phase-rich layer in the upper surface region and (B) a (U,Zr)O particle-rich layer at the inner region. Measurable concentrations of Sr and Ba were observed in layer (A) (approximately 1.7 times that in the layer (B)). According to thermodynamic analysis, (U,Zr)O
particle-rich layer at the inner region. Measurable concentrations of Sr and Ba were observed in layer (A) (approximately 1.7 times that in the layer (B)). According to thermodynamic analysis, (U,Zr)O is predicted to solidify, in advance, in the concrete-based melt around 2177
is predicted to solidify, in advance, in the concrete-based melt around 2177  C. Then, the residual melt is solidified as a silicate glass, and Sr and Ba are preferentially dissolved into the silicate glass. During the tests, (U,Zr)O
C. Then, the residual melt is solidified as a silicate glass, and Sr and Ba are preferentially dissolved into the silicate glass. During the tests, (U,Zr)O particles sank, in advance, in the melt because of its higher density, and the silicate glass phase relocated to the surface layer. On the other hand, silicate glass containing Sr and Ba is predicted to be hardly soluble in water and chemically stable.
particles sank, in advance, in the melt because of its higher density, and the silicate glass phase relocated to the surface layer. On the other hand, silicate glass containing Sr and Ba is predicted to be hardly soluble in water and chemically stable.
Sudo, Ayako; Meszaros, B.*; Poznyak, I.*; Sato, Takumi; Nagae, Yuji; Kurata, Masaki
Journal of Nuclear Materials, 533, p.152093_1 - 152093_8, 2020/05
Times Cited Count:4 Percentile:45.45(Materials Science, Multidisciplinary)Sudo, Ayako; Mizusako, Fumiki*; Hoshino, Kuniyoshi*; Sato, Takumi; Nagae, Yuji; Kurata, Masaki
Nihon Genshiryoku Gakkai Wabun Rombunshi, 18(3), p.111 - 118, 2019/08
Cooling rate of molten core materials during solidification significantly affects the segregation of major constituents of fuel debris. To understand general tendency of the segregation, liquefaction/solidification tests of simulated corium (UO , ZrO
, ZrO , FeO, B
, FeO, B C and sim-FP oxides) were performed. Simulated corium was heated up to 2600
C and sim-FP oxides) were performed. Simulated corium was heated up to 2600 C under Ar atmosphere and then cooled down with two different cooling processes; furnace cooling (average cooling rate is approximately 744
C under Ar atmosphere and then cooled down with two different cooling processes; furnace cooling (average cooling rate is approximately 744 C/min) and slow cooling (cooling rate in 2600
C/min) and slow cooling (cooling rate in 2600 C
C 2300
2300 C is 5
C is 5 C/min and in 2300
C/min and in 2300 C
C 1120
1120 C is approximately 788
C is approximately 788 C/min). Element analysis detected three oxide phases with different composition and one metal phase in both solidified samples. Solubility of FeO in these oxide phases was mostly fixed to be 12
C/min). Element analysis detected three oxide phases with different composition and one metal phase in both solidified samples. Solubility of FeO in these oxide phases was mostly fixed to be 12 5at% in both samples, which is in reasonable accordance with the value estimated from UO
5at% in both samples, which is in reasonable accordance with the value estimated from UO -ZrO
-ZrO -FeO phase diagrams. However, a significant grain-growth of one oxide phase, rich in Zr-oxide, was detected only in the slowly cooled sample. The composition of this particular oxide phase is comparable to the initial average composition. The condensation is considered to be caused by the connection of remaining liquid agglomerates during slow solidification.
-FeO phase diagrams. However, a significant grain-growth of one oxide phase, rich in Zr-oxide, was detected only in the slowly cooled sample. The composition of this particular oxide phase is comparable to the initial average composition. The condensation is considered to be caused by the connection of remaining liquid agglomerates during slow solidification.
Sudo, Ayako; Nishi, Tsuyoshi; Shirasu, Noriko; Takano, Masahide; Kurata, Masaki
Journal of Nuclear Science and Technology, 52(10), p.1308 - 1312, 2015/10
Times Cited Count:13 Percentile:73.22(Nuclear Science & Technology)For understanding the control blade degradation mechanism of BWR, the thermodynamic database for the fuel assembly materials is a useful tool. Although iron, boron, and carbon ternary system is a dominant phase diagram, phase relation data is not sufficient for the region in which the boron and carbon compositions are richer than the eutectic composition. The phase relations of three samples were analyzed by X-ray diffraction, scanning electron microscope and energy dispersed X-ray spectrometry. The results indicate that Fe (B,C) phase only exists in the intermediate region at 1273 K and that the solidus temperature widely maintains at about 1400 K for all three samples, which are different from the calculated data using previous thermodynamic database. The difference might be originated from the over-estimations of the interaction parameter between boron and carbon in Fe
(B,C) phase only exists in the intermediate region at 1273 K and that the solidus temperature widely maintains at about 1400 K for all three samples, which are different from the calculated data using previous thermodynamic database. The difference might be originated from the over-estimations of the interaction parameter between boron and carbon in Fe (B,C).
(B,C).
Sudo, Ayako; Shirasu, Noriko; Nishi, Tsuyoshi; Kurata, Masaki
no journal, ,
no abstracts in English
Sudo, Ayako; Nishi, Tsuyoshi; Shirasu, Noriko; Kurata, Masaki
no journal, ,
no abstracts in English
Sudo, Ayako; Nishi, Tsuyoshi; Shirasu, Noriko; Takano, Masahide; Kurata, Masaki
no journal, ,
Although Fe-B-C ternary system is a dominant phase diagram when considering the control blade degradation, phase relation data around eutectic composition were not sufficient. The phase relations of three Fe-B-C samples were analyzed by XRD and SEM/EDX and solidus were determined by DTA. The solidus detected in the present study was maintained at about 1400 K for all three samples, although preliminary calculation using conventional thermodynamic database estimated that solidus varies with roughly 50 K difference. The difference might be originated from the insufficient evaluation on the phase stability of Fe (B,C) in the database. The present results give useful information on improvement of iron-boron-carbon phase diagram.
(B,C) in the database. The present results give useful information on improvement of iron-boron-carbon phase diagram.
 specimens
specimensTakano, Masahide; Nishi, Tsuyoshi; Sudo, Ayako
no journal, ,
For the removal operation of fuel debris of the Fukushima Daiichi Nuclear Power Plant, some material properties of sintered (U,Zr)O solid solution specimens were measured. Their heat capacity and thermal conductivity were determined in the temperature range from room temperature to 1073 K. Micro Vickers hardness and electrical resistivity were also measured at room temperature as a function of U fraction. In addition to these material properties, the correlation between phases and O/M ratios of U-Zr-O system under oxidizing conditions was investigated. In the (U,Zr)
solid solution specimens were measured. Their heat capacity and thermal conductivity were determined in the temperature range from room temperature to 1073 K. Micro Vickers hardness and electrical resistivity were also measured at room temperature as a function of U fraction. In addition to these material properties, the correlation between phases and O/M ratios of U-Zr-O system under oxidizing conditions was investigated. In the (U,Zr)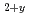 hyperstoichiometric region, the hypothetical O/U ratio in the solid solution was found to be independent of either U fraction and crystal structure. Further, three orthorhombic phases in the U-Zr-O system under more oxidizing conditions were identified, and their phase relationship was successfully defined.
hyperstoichiometric region, the hypothetical O/U ratio in the solid solution was found to be independent of either U fraction and crystal structure. Further, three orthorhombic phases in the U-Zr-O system under more oxidizing conditions were identified, and their phase relationship was successfully defined.
Takano, Masahide; Onozawa, Atsushi; Sudo, Ayako
no journal, ,
no abstracts in English
Sudo, Ayako; Onozawa, Atsushi; Takano, Masahide
no journal, ,
no abstracts in English
Sudo, Ayako
no journal, ,
no abstracts in English
Sudo, Ayako
no journal, ,
no abstracts in English
Sudo, Ayako; Onozawa, Atsushi; Takano, Masahide
no journal, ,
To characterize the layer structure and respective temperatures of MCCI products, MCCI experiments using a light-concentrating furnace were performed. As the core-melt constituents, a powder mixture of (U Zr
Zr )O
)O (No.1) and (U
(No.1) and (U Zr
Zr )O
)O /Zr/SUS316L/B
/Zr/SUS316L/B C (No.2) were compacted into tablets (10 mm in diameter) respectively. The tablet was placed on a cylindrical piece of basaltic concrete (25 mm in diameter). The light was concentrated on the tablet under argon gas flow and samples were heated. After heating, the vertical cross-section of the solidified sample piece was subjected to phase identification and elemental analysis by XRD and SEM/EDX. No.1 sample seemed to maintain original shape, but EDX analysis showed the inner region melted. This melt was identified as-melted (U,Zr)O
C (No.2) were compacted into tablets (10 mm in diameter) respectively. The tablet was placed on a cylindrical piece of basaltic concrete (25 mm in diameter). The light was concentrated on the tablet under argon gas flow and samples were heated. After heating, the vertical cross-section of the solidified sample piece was subjected to phase identification and elemental analysis by XRD and SEM/EDX. No.1 sample seemed to maintain original shape, but EDX analysis showed the inner region melted. This melt was identified as-melted (U,Zr)O particle and silicate glass containing U and Zr. Ca, Fe and Mg oxide were dissolved in the particle. UO
particle and silicate glass containing U and Zr. Ca, Fe and Mg oxide were dissolved in the particle. UO and ZrO
and ZrO were dissolved in the silicate glass. No.2 sample had same phases, but Fe-Cr oxide deposits from SS was identified.
were dissolved in the silicate glass. No.2 sample had same phases, but Fe-Cr oxide deposits from SS was identified.
Sudo, Ayako
no journal, ,
no abstracts in English
 simulated fuel debris
simulated fuel debrisTakano, Masahide; Onozawa, Atsushi; Sudo, Ayako
no journal, ,
no abstracts in English
Sudo, Ayako; Takano, Masahide; Onozawa, Atsushi
no journal, ,
To characterize the reaction layers with respective temperatures around core melt/concrete interface, we have performed MCCI experiments in laboratory scale by using a light-concentrating technique. As minor constituents, Gd O
O (burnable poison and FP), Mo-Ru-Rh-Pd alloy (FP), and sea salt were added in the simulated MCCI debris. The analyses of the top part of the sample identified as-melted (U,Zr)O
(burnable poison and FP), Mo-Ru-Rh-Pd alloy (FP), and sea salt were added in the simulated MCCI debris. The analyses of the top part of the sample identified as-melted (U,Zr)O particles precipitated in silicate glass containing U, Zr, Gd, Fe and Cr. Gd was included in both (U,Zr)O
particles precipitated in silicate glass containing U, Zr, Gd, Fe and Cr. Gd was included in both (U,Zr)O and silicate glass and Mo and the platinum group elements formed alloys with Fe-Ni-Mo-Ru-Rh-Pd. Although almost sea salt evaporated and deposited on the belljar, some S originating from sea salt resulted in precipitation of FeS-type sulfide in the alloy.
and silicate glass and Mo and the platinum group elements formed alloys with Fe-Ni-Mo-Ru-Rh-Pd. Although almost sea salt evaporated and deposited on the belljar, some S originating from sea salt resulted in precipitation of FeS-type sulfide in the alloy.
Sudo, Ayako; Takano, Masahide; Onozawa, Atsushi
no journal, ,
To characterize the reaction layers around core melt/concrete interface, MCCI experiments by using a light-concentrating technique was performed. As the main constituents of the core-melt, powder mixtures of ZrO , Zr, (U,Zr)O
, Zr, (U,Zr)O , stainless steel (SS), and B
, stainless steel (SS), and B C with various compositions were compacted into tablets. The tablet was placed on a concrete. Light from a lamp was concentrated on the tablet, and the vertical cross-section of the solidified sample was determined by XRD and SEM/EDX. The analyses identified 4 layers from top to bottom; (a) as-melted (U,Zr)O
C with various compositions were compacted into tablets. The tablet was placed on a concrete. Light from a lamp was concentrated on the tablet, and the vertical cross-section of the solidified sample was determined by XRD and SEM/EDX. The analyses identified 4 layers from top to bottom; (a) as-melted (U,Zr)O particles and silicate glass with U, (b) the silicate glass with U, (c) imperfectly melted concrete, and (d) dehydrated concrete. Unoxidized metal particles (Fe-Ni-Cr) also precipitated. Gd
particles and silicate glass with U, (b) the silicate glass with U, (c) imperfectly melted concrete, and (d) dehydrated concrete. Unoxidized metal particles (Fe-Ni-Cr) also precipitated. Gd O
O , Mo-Ru-Rh-Pd alloy, and sea salt were also added in the tablet. In this case Gd was included in both (U,Zr)O
, Mo-Ru-Rh-Pd alloy, and sea salt were also added in the tablet. In this case Gd was included in both (U,Zr)O and silicate glass, Mo and the platinum group elements formed alloys with Fe-Ni-Cr, and S originating from sea salt resulted in precipitation of FeS-type sulfide in the alloy.
and silicate glass, Mo and the platinum group elements formed alloys with Fe-Ni-Cr, and S originating from sea salt resulted in precipitation of FeS-type sulfide in the alloy.
Takano, Masahide; Onozawa, Atsushi; Sudo, Ayako
no journal, ,
To understand the characteristics of MCCI products in Fukushima Daiichi Nuclear Power Station, the simulated MCCI products in laboratory scale were prepared by arc melting of compacted powder mixtures of core materials and concrete. Stainless steel, boron carbide, metallic zirconium, (U,Zr)O , GdO
, GdO , and platinum group elements were selected as the core materials. Phases, morphology, and micro hardness were analyzed on cross-section of the solidified specimens. The specimens consisted of oxide part (MO
, and platinum group elements were selected as the core materials. Phases, morphology, and micro hardness were analyzed on cross-section of the solidified specimens. The specimens consisted of oxide part (MO corium and silicate glass or Al-Ca-O) and metallic part (alloys and borides). The phase relationships in the MCCI products were found to be dominated by the initial concrete/Zr mixing ratio, because the dehydration of concrete is the main oxidation factor and the metallic zirconium acts as a strong reductant. Micro hardness of main phases are 7 GPa for silicate glass, 13-15 GPa for (U,Zr,Gd,Ca)O
corium and silicate glass or Al-Ca-O) and metallic part (alloys and borides). The phase relationships in the MCCI products were found to be dominated by the initial concrete/Zr mixing ratio, because the dehydration of concrete is the main oxidation factor and the metallic zirconium acts as a strong reductant. Micro hardness of main phases are 7 GPa for silicate glass, 13-15 GPa for (U,Zr,Gd,Ca)O corium, and 25 GPa for ZrB
corium, and 25 GPa for ZrB and ferrous borides, respectively.
and ferrous borides, respectively.