Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Yokoyama, Keisuke; Watanabe, Masashi; Onishi, Takashi; Yano, Yasuhide; Tokoro, Daishiro*; Sugata, Hiromasa*; Kato, Masato*
JAEA-Research 2025-002, 18 Pages, 2025/05
It is advocated as a development target of fast reactors (FRs) to allow for the of use of mixed oxide (MOX) fuels containing minor actinide (MA) separated and recovered from spent fuels with the aim of reducing the volume and toxicity of high-level radioactive waste generated from nuclear reactors. In the development of MAMOX fuels, it is important behavior to understand the thermal properties such as thermal conductivity for fuel design and analysis of the irradiation. However, there are only a few reports on the thermal properties of MA-MOX fuels, and neither the effects of MA contents nor of oxygen non-stoichiometry in MOX fuels on their thermal conductivities have been fully understood. In this study, the thermal conductivities of MOX fuels with up to 15% Am content were measured at near-stoichiometric composition and the relationship between thermal conductivity and Am content was evaluated. Moreover, the thermal conductivities of Am-doped UO fuels were also measured and evaluated by comparison with Am-MOX to evaluate the effect of Am content. The fuel samples used in this study were three types of MOX with a Pu content of 30% and different Am contents (5%, 10%, and 15%), and UO
fuels were also measured and evaluated by comparison with Am-MOX to evaluate the effect of Am content. The fuel samples used in this study were three types of MOX with a Pu content of 30% and different Am contents (5%, 10%, and 15%), and UO containing 15% Am. The thermal conductivities of specimens were calculated from the thermal diffusivities measured by the laser flash method, the density of the specimens and, the heat capacity at constant pressure. The oxygen partial pressure during the measurement was controlled at that of the targeted near-stoichiometric composition. The thermal conductivities of all specimens exhibited a decline with increasing temperature and Am content, with a particularly pronounced reduction observed below 1,173 K. The results of the classical phonon scattering model analysis of the measured thermal conductivities showed that the effect of lattice strain due to the Am addition was significant on the thermal resistivity change, and the effect was comparable for both MOX and UO
containing 15% Am. The thermal conductivities of specimens were calculated from the thermal diffusivities measured by the laser flash method, the density of the specimens and, the heat capacity at constant pressure. The oxygen partial pressure during the measurement was controlled at that of the targeted near-stoichiometric composition. The thermal conductivities of all specimens exhibited a decline with increasing temperature and Am content, with a particularly pronounced reduction observed below 1,173 K. The results of the classical phonon scattering model analysis of the measured thermal conductivities showed that the effect of lattice strain due to the Am addition was significant on the thermal resistivity change, and the effect was comparable for both MOX and UO .
.
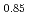 Am
Am O
O at 1473, 1573, and 1673 K
at 1473, 1573, and 1673 KWatanabe, Masashi; Yokoyama, Keisuke; Vauchy, R.; Kato, Masato; Sugata, Hiromasa*; Seki, Takayuki*; Hino, Tetsushi*
Journal of Nuclear Materials, 599, p.155232_1 - 155232_5, 2024/10
Times Cited Count:2 Percentile:75.80(Materials Science, Multidisciplinary)Oxygen potential data of U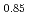 Am
Am O
O were measured at 1473, 1573, and 1673 K by thermogravimetry. In U
were measured at 1473, 1573, and 1673 K by thermogravimetry. In U An
An O
O , where An stands for Pu or Am, and for a given value of y and Oxygen/Metal ratio, the oxygen potential of U
, where An stands for Pu or Am, and for a given value of y and Oxygen/Metal ratio, the oxygen potential of U Am
Am O
O is higher than that of U
is higher than that of U Pu
Pu O
O . The valence of cations in the hypostoichiometric region is similar to that of Nd-doped UO
. The valence of cations in the hypostoichiometric region is similar to that of Nd-doped UO . At the stoichiometric composition, it is estimated to be Am
. At the stoichiometric composition, it is estimated to be Am , U
, U , and U
, and U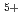 (for charge compensation of Am
(for charge compensation of Am ). The experimental data were analyzed using a defect chemistry model, and a relationship connecting the oxygen-to-metal ratio, the temperature, and the equilibrium oxygen partial pressure was proposed.
). The experimental data were analyzed using a defect chemistry model, and a relationship connecting the oxygen-to-metal ratio, the temperature, and the equilibrium oxygen partial pressure was proposed.
 -PuO
-PuO -ZrO
-ZrO pseudo-ternary system
pseudo-ternary systemMorimoto, Kyoichi; Hirooka, Shun; Akashi, Masatoshi; Watanabe, Masashi; Sugata, Hiromasa*
Journal of Nuclear Science and Technology, 52(10), p.1247 - 1252, 2015/10
Times Cited Count:4 Percentile:30.26(Nuclear Science & Technology)As a part of decommissioning plan of the damaged reactors at Fukushima Daiichi Nuclear Power Plant, some strategies for removing of debris from the reactors are discussed. In these considerations, it is necessary to predict a melt progression during the severe accident based on theoretical evidences. Melting temperature is one of the most important thermal characteristics to analyse a melt progression during the severe accident. In this study, the melting temperatures of specimens of U, Pu and Zr mixed oxide prepared as simulated debris were measured by the thermal arrest technique. From the results of this measurement, the influences of Pu and Zr
and Zr contents on the melting temperature of the simulated debris were evaluated.
contents on the melting temperature of the simulated debris were evaluated.
 -MOX system
-MOX systemUchida, Teppei; Hirooka, Shun; Sugata, Hiromasa*; Shibata, Katsuya*; Sato, Daisuke*; Kato, Masato; Morimoto, Kyoichi
Proceedings of International Nuclear Fuel Cycle Conference; Nuclear Energy at a Crossroads (GLOBAL 2013) (CD-ROM), p.1549 - 1553, 2013/09
Kato, Masato; Komeno, Akira; Uno, Hiroki*; Sugata, Hiromasa*; Nakae, Nobuo; Konashi, Kenji*; Kashimura, Motoaki
Journal of Nuclear Materials, 393(1), p.134 - 140, 2009/08
Times Cited Count:45 Percentile:92.64(Materials Science, Multidisciplinary)In plutonium compounds, the lattice parameter increases due to self-radiation damage by  -decay of plutonium isotopes. The lattice parameter change and its thermal recovery in plutonium and uranium mixed dioxide (MOX) were studied. The lattice parameter for samples of MOX powders and pellets that had been left in the air for up to 32 years was measured. The lattice parameter increased and was saturated at about 0.29%. The change in lattice parameter was formulated as a function of self-radiation dose. Three stages in the thermal recovery of the damage were observed in temperature ranges of below 673K, 673-1073K and above 1073K. The activation energies in each recovery stage were estimated to be 0.12 eV, 0.73 eV and 1.2 eV, respectively, and the corresponding mechanism for each stage was considered to be the recovery of the anion Frenkel defect, the cation Frenkel defect and a defect connected with helium, respectively.
-decay of plutonium isotopes. The lattice parameter change and its thermal recovery in plutonium and uranium mixed dioxide (MOX) were studied. The lattice parameter for samples of MOX powders and pellets that had been left in the air for up to 32 years was measured. The lattice parameter increased and was saturated at about 0.29%. The change in lattice parameter was formulated as a function of self-radiation dose. Three stages in the thermal recovery of the damage were observed in temperature ranges of below 673K, 673-1073K and above 1073K. The activation energies in each recovery stage were estimated to be 0.12 eV, 0.73 eV and 1.2 eV, respectively, and the corresponding mechanism for each stage was considered to be the recovery of the anion Frenkel defect, the cation Frenkel defect and a defect connected with helium, respectively.
Kato, Masato; Morimoto, Kyoichi; Nakamichi, Shinya; Sugata, Hiromasa*; Konashi, Kenji*; Kashimura, Motoaki; Abe, Tomoyuki
Nihon Genshiryoku Gakkai Wabun Rombunshi, 7(4), p.420 - 428, 2008/12
The melting temperatures of MOX for fast reactor fuel were investigated as functions of Pu content, Am content and oxygen-to-metal (O/M) ratio using thermal arrest technique. Rhenium inner was used for the measurement to prevent the reaction between the sample and capsule materials. The solidus temperatures decreased with increasing Pu and Am content and increased with decreasing O/M ratio. It is considered that the maximum temperature in U-Pu-O system varies in hypostoichiometric composition region. The melting temperatures were evaluated by ideal solid solution model in UO -PuO
-PuO -AmO
-AmO -PuO
-PuO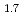 system, and the model was derived for calculating solidus and liquidus temperature. The derived model reproduced the experimental data with
system, and the model was derived for calculating solidus and liquidus temperature. The derived model reproduced the experimental data with  25 K.
25 K.
Kato, Masato; Morimoto, Kyoichi; Sugata, Hiromasa*; Konashi, Kenji*; Kashimura, Motoaki; Abe, Tomoyuki
Journal of Alloys and Compounds, 452(1), p.48 - 53, 2008/03
Times Cited Count:32 Percentile:78.03(Chemistry, Physical)Plutonium and uranium mixed oxide has been developed as a fuel of a fast reactor. The maximum temperature of the fuel pellet is limited within a design criterion to prevent fuel melting. So, the melting points of the mixed oxide have been investigated since the development of fast reactor started. However the measured data are limited. In this work, the melting points of (U1-yPuy)O (y: 0, 0.12, 0.2, 0.3, 0.4) were measured by the thermal arrest method. The evaluated melting point of this study underestimates in case of MOX with high Pu contents of 30% and 40%. The solidus of UO
(y: 0, 0.12, 0.2, 0.3, 0.4) were measured by the thermal arrest method. The evaluated melting point of this study underestimates in case of MOX with high Pu contents of 30% and 40%. The solidus of UO , (Pu
, (Pu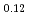 U
U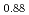 )O
)O and (Pu
and (Pu U
U )O
)O were determined to be 3128K, 3077K and 3052K, respectively. The solidus temperature of hypostoichiometric MOX slightly increased with decreasing O/M.
were determined to be 3128K, 3077K and 3052K, respectively. The solidus temperature of hypostoichiometric MOX slightly increased with decreasing O/M.
 -PuO
-PuO system
systemKato, Masato; Morimoto, Kyoichi; Sugata, Hiromasa*; Konashi, Kenji*; Kashimura, Motoaki; Abe, Tomoyuki
Journal of Nuclear Materials, 373(1-3), p.237 - 245, 2008/02
Times Cited Count:64 Percentile:96.04(Materials Science, Multidisciplinary)The melting of plutonium and uranium mixed oxide (MOX) containing Pu of more than 30% was investigated using a tungsten capsule and a rhenium inner capsule. In the conventional measurement of MOX in the tungsten capsule, a liquid phase of tungsten and plutonium oxide appeared in the MOX during melting. This liquid phase was found to have an effect on the measurement of melting point. Therefore the rhenium inner capsule was used to avoid the effect. The solidus and liquidus temperatures in the UO -PuO
-PuO system were decided from the MOX data measured using the rhenium capsule, and the effect of the Am content on the solidus temperature was evaluated. The variation of the solidus and liquidus temperatures in the UO
system were decided from the MOX data measured using the rhenium capsule, and the effect of the Am content on the solidus temperature was evaluated. The variation of the solidus and liquidus temperatures in the UO -PuO
-PuO -AmO
-AmO ternary system was represented to an accuracy of
ternary system was represented to an accuracy of  =
= 9K and
9K and  =
= 16K, respectively, by the ideal solution model.
16K, respectively, by the ideal solution model.
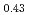 Am
Am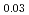 U
U )O
)O
Nakamichi, Shinya; Kato, Masato; Morimoto, Kyoichi; Sugata, Hiromasa*; Kashimura, Motoaki; Abe, Tomoyuki
Transactions of the American Nuclear Society, 96(1), p.191 - 192, 2007/06
JAEA has developed plutonium and uranium mixed oxide (MOX) containing 20-32%Pu content as a fuel of the fast breeder reactor. During irradiation, large temperature gradient in radial direction of a fuel pellet causes redistribution of Pu and U, and the Pu content increases to about 43% at the pellet center. The maximum temperature of the fuel pellet during irradiation is limited within the design criterion to prevent fuel melting. So, it is important to evaluate melting points of MOX containing 43%Pu. In this work, it is confirmed that the MOX with 43%Pu content is not melted by heat treatment just below the melting point which was determined by thermal arrest technique using Re inner capsule. The MOX specimen with 43%Pu content was heated at 2978K for 40s using Re inner capsule. Optical micrograph and XRD results show the specimen was heated at the temperature less than solidus temperature. So it was confirmed that (Pu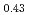 Am
Am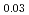 U
U )O
)O was solid phase at 2978K
was solid phase at 2978K 20K.
20K.
Kato, Masato; Morimoto, Kyoichi; Sugata, Hiromasa*; Konashi, Kenji*; Kashimura, Motoaki; Abe, Tomoyuki
Transactions of the American Nuclear Society, 96(1), p.193 - 194, 2007/06
Melting point of a nuclear fuel is one of the important physical properties for its development, because it limits maximum temperature of the fuel during operation. A rhenium inner capsule was used to prevent the reaction with capsule for measuring melting points of MOX. In this work melting points of MOX with 40% and 46%Pu were investigated as a function of an O/M ratio using Re inner, and the effect of the O/M ratio on the melting points was evaluated. The solidus and liquidus temperatures in (Pu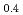 U
U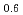 )O
)O and (Pu
and (Pu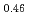 U
U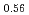 )O
)O were measured by thermal arrest method. It was observed that the melting points in the both samples increased with a decrease of the O/M from 2.00, and their data were 50-100K higher than existing data measured in previous works which were measured with W capsule.
were measured by thermal arrest method. It was observed that the melting points in the both samples increased with a decrease of the O/M from 2.00, and their data were 50-100K higher than existing data measured in previous works which were measured with W capsule.
Kato, Masato; Morimoto, Kyoichi; Komeno, Akira; Nakamichi, Shinya; Kashimura, Motoaki; Abe, Tomoyuki; Uno, Hiroki*; Ogasawara, Masahiro*; Tamura, Tetsuya*; Sugata, Hiromasa*; et al.
JAEA-Technology 2006-049, 32 Pages, 2006/10
Japan Atomic Energy Agency has developed a fast breeder reactor(FBR), and plutonium and uranium mixed oxide (MOX) having low density and 20-30%Pu content has used as a fuel of the FBR, Monju. In plutonium, Americium has been accumulated during long-term storage, and Am content will be increasing up to 2-3% in the MOX. It is essential to evaluate the influence of Am content on physical properties of MOX on the development of FBR in the future. In this study melting points and thermal conductivities which are important data on the fuel design were measured systematically in wide range of composition, and the effects of Am accumulated were evaluated. The solidus temperatures of MOX were measured as a function of Pu content, oxygen to metal ratio(O/M) and Am content using thermal arrest technique. The sample was sealed in a tungsten capsule in vacuum for measuring solidus temperature. In the measurements of MOX with Pu content of more than 30%, a rhenium inner capsule was used to prevent the reaction between MOX and tungsten. In the results, it was confirmed that the melting points of MOX decrease with as an increase of Pu content and increase slightly with a decrease of O/M ratio. The effect of Am content on the fuel design was negligible small in the range of Am content up to 3%. Thermal conductivities of MOX were evaluated from thermal diffusivity measured by laser flash method and heat capacity calculated by Nuemann- Kopp's law. The thermal conductivity of MOX decreased slightly in the temperature of less than 1173K with increasing Am content. The effect of Am accumulated in long-term storage fuel was evaluated from melting points and thermal conductivities measured in this study. It is concluded that the increase of Am in the fuel barely affect the fuel design in the range of less than 3%Am content.
Kato, Masato; Sugata, Hiromasa*; Endo, Hideo
JNC TN8400 2002-019, 41 Pages, 2003/03
In Plutonium compounds the self-radiation induces expansion of lattice parameter and change in thermal conductivity. The expansion of the lattice parameter and thermal recovery of radiation damage in plutonium and uranium mixed dioxide (MOX) were studied in this paper. MOX powder had been kept in an ambient atmosphere for about two years. The lattice parameter of the powder was expanded up to about 0.23%. The change in lattice parameter was formulated as a function of amount of self-radiation. Three thermal recovery stage of radiation damage were observed in temperature ranges below 400 C, 400-800
C, 400-800 C and above 800
C and above 800 C. The recovery rate of three stages in total lattice expansion was about 25%, 55% and 20%, respectively, and activation energy in each recovery was estimated to be 0.14 eV 0.54 eV and 1.1 eV.
C. The recovery rate of three stages in total lattice expansion was about 25%, 55% and 20%, respectively, and activation energy in each recovery was estimated to be 0.14 eV 0.54 eV and 1.1 eV.

Morimoto, Kyoichi; Kato, Masato; Nishiyama, Motokuni; Endo, Hideo; Kono, Shusaku; Uno, Hiroki*; Tamura, Tetsuya*; Sugata, Hiromasa*
JNC TN8400 2003-011, 32 Pages, 2003/01
MOX fuel containing Neptunium is being developed as candidate fuel for an advanced nuclear fuel recycle. In this report, influence of Np on the sintering behavior, phase separation behavior of MOX fuel pellets and the homogeneity of MOX fuel pellets were evaluated. It was observed that the high Np containing pellets had a low sintered density and the microstructure changes of the pellets during the sintering were slow compared with MOX without Np. The pellets were also analyzed via Ceramography, X-ray diffraction measurement and an electron probe microanalysis. The phase separation behavior of MOX with Np was similar to that of MOX. The homogeneity of the pellet produced with this experiment was acceptable to the fuel specification.
Morimoto, Kyoichi; Kato, Masato; Kono, Shusaku; Sugata, Hiromasa*; Sunaoshi, Takeo*
Abstract, PB63, (PB63), 0 Pages, 2003/00
None
Kato, Masato; Sugata, Hiromasa*; Takahashi, Kuniaki; Kamimura, Katsuichiro
PNC TN8410 97-018, 38 Pages, 1997/01
None
 irradiation
irradiationKomeno, Akira; Kato, Masato; Morimoto, Kyoichi; Kashimura, Motoaki; Sugata, Hiromasa*; Shibata, Katsuya*; Tamura, Tetsuya*; Uno, Hiroki*
no journal, ,
no abstracts in English
 and Zircalloy-2, 2; Melting point measurement and microstructure
and Zircalloy-2, 2; Melting point measurement and microstructureUchida, Teppei; Sugata, Hiromasa*; Shibata, Katsuya*; Sato, Daisuke*; Morimoto, Kyoichi
no journal, ,
no abstracts in English

Tsuchimochi, Ryota; Kato, Masato; Hirooka, Shun; Matsumoto, Taku; Uno, Hiroki*; Ogasawara, Masahiro*; Sugata, Hiromasa*
no journal, ,
Thermal expansion and specific heat of CaF , which forms the same crystal structure as actinide oxides, were measured by using high-temperature X-ray diffractmeter and differential scanning calorimetry. We confirmed that the specific heat of CaF
, which forms the same crystal structure as actinide oxides, were measured by using high-temperature X-ray diffractmeter and differential scanning calorimetry. We confirmed that the specific heat of CaF explicitly shows the effect of excitation on top of the heat capacity at constant volume and dilatational term. The analysis of the specific heat will be discussed together with the measured data.
explicitly shows the effect of excitation on top of the heat capacity at constant volume and dilatational term. The analysis of the specific heat will be discussed together with the measured data.
Kato, Masato; Morimoto, Kyoichi; Kashimura, Motoaki; Abe, Tomoyuki; Uno, Hiroki*; Sugata, Hiromasa*; Tamura, Tetsuya*; Shibata, Katsuya*
no journal, ,
no abstracts in English
Komeno, Akira; Kato, Masato; Morimoto, Kyoichi; Kashimura, Motoaki; Sugata, Hiromasa*; Shibata, Katsuya*; Uno, Hiroki*; Tamura, Tetsuya*
no journal, ,
no abstracts in English