Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
 Sr measurement
Sr measurementHorita, Takuma; Asai, Shiho; Konda, Miki; Hanzawa, Yukiko; Saito, Kyoichi*; Fujiwara, Kunio*; Sugo, Takanobu*; Kitatsuji, Yoshihiro
Bunseki Kagaku, 66(3), p.189 - 193, 2017/03
Times Cited Count:1 Percentile:3.41(Chemistry, Analytical)A Sr-selective adsorption fiber was prepared for rapid analysis of  Sr content by using radiation-induced emulsion graft polymerization and subsequent chemical modification. A polyethylene fiber with a diameter of 13
Sr content by using radiation-induced emulsion graft polymerization and subsequent chemical modification. A polyethylene fiber with a diameter of 13  m was first immersed in a methanol solution of an epoxy-group-containing vinyl monomer, glycidyl methacrylate (GMA), and polyoxyethylene sorbitol ester (Tween20) as a surfactant for graft-polymerization of GMA. Octadecylamine was then bound to a polymer chain extending from the fiber surface providing hydrophobicity to the polymer chain. Dicyclohexano-18-crown-6 (DCH18C6) was finally impregnated onto the polymer chain via a hydrophobic interaction between the octadecyl moiety of the polymer chain and the cyclohexyl moiety of DCH18C6. The fiber surface structure, characterized by DCH18C6 molecules loosely entangled with polymer chains, afforded realizes the rapid and selective adsorption of Sr ions with an adsorption rate approximately 100 times higher than that of a commercially available Sr-selective resin (Sr Resin).
m was first immersed in a methanol solution of an epoxy-group-containing vinyl monomer, glycidyl methacrylate (GMA), and polyoxyethylene sorbitol ester (Tween20) as a surfactant for graft-polymerization of GMA. Octadecylamine was then bound to a polymer chain extending from the fiber surface providing hydrophobicity to the polymer chain. Dicyclohexano-18-crown-6 (DCH18C6) was finally impregnated onto the polymer chain via a hydrophobic interaction between the octadecyl moiety of the polymer chain and the cyclohexyl moiety of DCH18C6. The fiber surface structure, characterized by DCH18C6 molecules loosely entangled with polymer chains, afforded realizes the rapid and selective adsorption of Sr ions with an adsorption rate approximately 100 times higher than that of a commercially available Sr-selective resin (Sr Resin).
Someya, Takaaki*; Asai, Shiho; Fujiwara, Kunio*; Sugo, Takanobu*; Umeno, Daisuke*; Saito, Kyoichi*
Nihon Kaisui Gakkai-Shi, 69(1), p.42 - 48, 2015/02
A large amount of seriously contaminated sea water with radioactive Cs has been reserved in semi-enclosed coastal sea area which is separated by silt fences and embankments. Insoluble cobalt ferrocyanide (Co-FC) microparticles-impregnated fiber was developed for removing Cs from the contaminated sea water. The resultant Co-FC-impregnated fiber was immersed in either nonradioactive or radioactive Cs solution. The adsorption isotherm well correlated with a Langmuir-type equation. In addition, mass-transfer capacity coefficients were determined by fitting the experimental data of the rate of Cs adsorption onto the Co-FC-impregnated fiber to theoretical adsorption curves based on the Cs concentration difference between the bulk and the interface in seawater as a driving force of the overall adsorption rate. Decontamination factors as functions of fiber weight and the contact time required for the removal of cesium ions from the contaminated seawater in a closed area were estimated.
Ishihara, Ryo*; Fujiwara, Kunio*; Harayama, Takato*; Okamura, Yusuke*; Uchiyama, Shoichiro*; Sugiyama, Mai*; Someya, Takaaki*; Amakai, Wataru*; Umino, Satoshi*; Ono, Tsubasa*; et al.
Journal of Nuclear Science and Technology, 48(10), p.1281 - 1284, 2011/10
Times Cited Count:44 Percentile:94.73(Nuclear Science & Technology)Sawaki, Kenta*; Asai, Shiho; Watanabe, Kazuo; Sugo, Takanobu*; Saito, Kyoichi*
Maku, 33(1), p.32 - 38, 2008/01
TOPO, tri-n-octylphosphine oxide, as a neutral extractant was impregnated to the hydrophobic group of the polymer chain grafted onto a porous hollow-fiber membrane for an efficient metal-ion collection. A diol group,i.e., adjacent hydroxyl group was introduced into poly-glycidyl methacrylate(GMA) chain grafted onto the porous hollow-fiber membrane via the epoxy-ring-opening reaction with water, followed by a reaction of the remaining epoxy group with octadecanethiol (C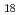 H
H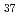 SH) to form an octadecanethiol group. The diol group reduced the amount of TOPO coagulatedon the graft chain, whereas the interaction of the octadecanethiol group of the graft chain with the hydrophobic part of TOPO enabled the impregnation of TOPO to the graft chain. A 1 mmol-Bi/L bismuth chloride solution was forced to permeate through the pores of the TOPO-impregnated porous hollow-fiber membrane with a density of TOPO impregnated of 1.3 mol/kg of the GMA-grafted porous hollow-fiber membrane and the pure water flux of 0.7 m/h at 0.1 MPa and 298 K. The equilibrium binding capacity of the membrane for bismuth ions was 0.22 mol/kg of the TOPO-impregnated porous hollow-fiber membrane, which was equivalent to a binding ratio of bismuth ion to impregnated TOPO of 1.0.
SH) to form an octadecanethiol group. The diol group reduced the amount of TOPO coagulatedon the graft chain, whereas the interaction of the octadecanethiol group of the graft chain with the hydrophobic part of TOPO enabled the impregnation of TOPO to the graft chain. A 1 mmol-Bi/L bismuth chloride solution was forced to permeate through the pores of the TOPO-impregnated porous hollow-fiber membrane with a density of TOPO impregnated of 1.3 mol/kg of the GMA-grafted porous hollow-fiber membrane and the pure water flux of 0.7 m/h at 0.1 MPa and 298 K. The equilibrium binding capacity of the membrane for bismuth ions was 0.22 mol/kg of the TOPO-impregnated porous hollow-fiber membrane, which was equivalent to a binding ratio of bismuth ion to impregnated TOPO of 1.0.
Asai, Shiho; Magara, Masaaki; Sakurai, Satoshi; Shinohara, Nobuo; Saito, Kyoichi*; Sugo, Takanobu*
Nihon Ion Kokan Gakkai-Shi, 18(4), p.486 - 491, 2007/10
Ishihara, Ryo*; Umeno, Daisuke*; Saito, Kyoichi*; Asai, Shiho; Sakurai, Satoshi; Shinohara, Nobuo; Sugo, Takanobu*
Nihon Ion Kokan Gakkai-Shi, 18(4), p.480 - 485, 2007/10
Asai, Shiho; Watanabe, Kazuo; Sugo, Takanobu*; Saito, Kyoichi*
Maku, 32(3), p.168 - 174, 2007/05
no abstracts in English
Sawaki, Kenta*; Domon, Sayaka*; Asai, Shiho; Watanabe, Kazuo; Sugo, Takanobu*; Saito, Kyoichi*
Maku, 32(2), p.109 - 115, 2007/03
no abstracts in English
Asai, Shiho; Watanabe, Kazuo; Saito, Kyoichi*; Sugo, Takanobu*
Journal of Membrane Science, 281(1-2), p.195 - 202, 2006/09
Times Cited Count:13 Percentile:44.25(Engineering, Chemical)Aliquat 336, tri- -octylmethylammonium chloride, was impregnated into the polymer chain grafted onto a porous membrane. 6-aminohexanoic acid and octadecylamine were introduced into the epoxy group of the graft chain. The amount of impregnated Aliquat 336 was 1.2 mol per kg of the GMA-grafted porous membrane. The coexistence of 6-aminohexanoic acid and octadecylamino groups enabled the high-density and stable impregnation of Aliquat 336 to the graft chain. Palladium chloride dissolved in 1 M hydrochloric acid was forced to permeate through the pores of the Aliquat 336-impregnated porous membrane. The binding efficiency was found to be 69%.
-octylmethylammonium chloride, was impregnated into the polymer chain grafted onto a porous membrane. 6-aminohexanoic acid and octadecylamine were introduced into the epoxy group of the graft chain. The amount of impregnated Aliquat 336 was 1.2 mol per kg of the GMA-grafted porous membrane. The coexistence of 6-aminohexanoic acid and octadecylamino groups enabled the high-density and stable impregnation of Aliquat 336 to the graft chain. Palladium chloride dissolved in 1 M hydrochloric acid was forced to permeate through the pores of the Aliquat 336-impregnated porous membrane. The binding efficiency was found to be 69%.
Asai, Shiho; Watanabe, Kazuo; Sugo, Takanobu*; Saito, Kyoichi*
Journal of Chromatography A, 1094(1-2), p.158 - 164, 2005/11
Times Cited Count:23 Percentile:55.44(Biochemical Research Methods)The analysis of radioactive species in radioactive wastes is essential to the safe and economical disposal of such wastes. Among radioactive species, alpha- and beta-emitting nuclides should be purified prior to various radiometric determinations. To overcome the disadvantages of the conventional separation techniques, we have proposed functional porous hollow-fiber membranes that achieve a high speed operation assisted by convective flow. Stable immobilization in aqueous media is ensured by the hydrophobic interaction between the hydrophobic moiety of the extractant and octadecyl part of octadecylamino group. In this study, HDEHP, which shows the selectivity for rare earth elements, such as yttrium, was immobilized onto the porous membrane. The amount of immobilized HDEHP increased with increasing molar conversion. This can be explained by the fact that an increase in the C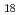 NH group allows the polymer brush to extend itself due to electrostatic repulsion originating from the amino part of the C
NH group allows the polymer brush to extend itself due to electrostatic repulsion originating from the amino part of the C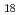 NH group.
NH group.
Domon, Sayaka*; Asai, Shiho; Saito, Kyoichi*; Watanabe, Kazuo; Sugo, Takanobu*
Journal of Membrane Science, 262(1-2), p.153 - 158, 2005/10
Times Cited Count:16 Percentile:49.92(Engineering, Chemical)no abstracts in English
Seko, Noriaki; Kasai, Noboru; Tamada, Masao; Hasegawa, Shin; Katakai, Akio; Sugo, Takanobu*
JAERI-Tech 2004-076, 78 Pages, 2005/01
In September 1999, we have soaked 200 kg of fibrous amidoxime adsorbents, synthesized by radiation-induced graft polymerization, into seawater to evaluate their performance. Fractional elution facility was set effectively to elute the rare metals on adsorbents in Mutsu-Establishment. This facility consists of two parts of pre-washing and elution. The present report dealt with planning, manufacture and setting of fractional facility. Marine organism and slime on adsorbent cassette (290 290
290 160 mm) were washed out and every 72 cassettes were set in elution unit (1210
160 mm) were washed out and every 72 cassettes were set in elution unit (1210 1210
1210 H1460 mm) with nonwoven materials as a packing to avoid elution loss. In the elution process alkaline and alkaline earth metals were eluted with low concentration hydrochloric acid (0.01M) and rare metals were eluted with high concentration (0.5M) after the packing of elution unit into fractional elution facility.
H1460 mm) with nonwoven materials as a packing to avoid elution loss. In the elution process alkaline and alkaline earth metals were eluted with low concentration hydrochloric acid (0.01M) and rare metals were eluted with high concentration (0.5M) after the packing of elution unit into fractional elution facility.
Seko, Noriaki; Takeda, Hayato*; Kasai, Noboru; Tamada, Masao; Hasegawa, Shin; Katakai, Akio; Sugo, Takanobu*
JAERI-Tech 2004-075, 51 Pages, 2005/01
Fibrous adsorbent which is synthesized by radiation induced graft polymerization on the trunk polymers such as polymer nonwoven fabrics and woven cloths exhibits an excellent selective adsorption against heavy metal ions and toxic gases at extremely low concentrations. Two equipments were installed to synthesize the metal-ion and gas adsorbents by means of the radiation-induced graft polymerization in the liquid phase and the dipping, respectively. In the reaction chamber of the liquid phase reactor, the oxygen decreased to 100ppm. The inside temperature raised to 80 C. These characteristics satisfied the specification. The fabric transport can regulate the rate in the range from 1 to 10m/min. The reactor for the dip grafting could reduce the inside oxygen to 100ppm and inside temperature could reach to 80
C. These characteristics satisfied the specification. The fabric transport can regulate the rate in the range from 1 to 10m/min. The reactor for the dip grafting could reduce the inside oxygen to 100ppm and inside temperature could reach to 80  C. The grafting of GMA was carried out as a characteristic test. The degree of grafting was controlled in the range from 40 to 70%.
C. The grafting of GMA was carried out as a characteristic test. The degree of grafting was controlled in the range from 40 to 70%.
Jo, Akinori*; Okada, Kenji*; Tamada, Masao; Kume, Tamikazu; Sugo, Takanobu; Tazaki, Masato*
Chemistry for the Protection of the Environment 4; Environmental Science Research, Vol. 59, p.49 - 62, 2005/00
Bifunctional cation exchange fibers were synthesized by co-grafting of chloromethylstylene and styrene. on polyethylene-coated polypropylene fibers. The grafted fibers were fictionalized by Arbuzov reaction, suffonation, and acid hydrolysis. Batchwise evaluation of metal ion selectivity clarified that the bifunctional fiber exhibited cooperative recognition of metal ions by both functional groups. The bifunctional fiber took up Pb(II) more rapidly than the monofunctional phosphoric acid fiber and commercial resin adsorbent. Column-mode experiment revealed that flow rate was independent of break through profiles of Pb(II) up to flow rate of 900 h in space velocity.
in space velocity.
Asai, Shiho; Watanabe, Kazuo; Sugo, Takanobu*; Saito, Kyoichi*
Separation Science and Technology, 40(16), p.3349 - 3364, 2005/00
Times Cited Count:3 Percentile:20.23(Chemistry, Multidisciplinary)Poly-glycidyl methacrylate (GMA) was appended onto a porous membrane of a hollow-fiber form. Octadecylamine was added to the epoxy group of the polymer brush at a maximum molar conversion of the epoxy group into the octadecylamino group of 59%. Bis(2,4,4,-trimethylpentyl)phosphinic acid (Cyanex 272), was impregnated on the octadecylamino group by immersion of the octadecylamine-added porous membrane. The phosphinic acid moiety of Cyanex 272 was attracted by the amino part of the octadecylamino group of the polymer brush to swell the entire volume of the porous membrane. The impregnated Cyanex 272 was repositioned on the charged polymer brush in response to the properties of surrounding liquids. The hydrophobic interaction enables the hydrophobic moiety of Cyanex 272 to associate with the octadecyl part of the octadecylamino group of the polymer brush to capture zinc ions. The binding efficiency of the Cyanex 272-impregnated polymer brush for zinc ion was as high as 93%.
Jo, Akinori*; Kugara, J.*; Trobradovic, H.*; Yamabe, Kazunori*; Sugo, Takanobu; Tamada, Masao; Kume, Tamikazu
Industrial & Engineering Chemistry Research, 43(7), p.1599 - 1607, 2004/03
Times Cited Count:28 Percentile:67.34(Engineering, Chemical)Fibrous iminodiacetic acid cheating cation exchangers were derived from chloromethylstyrene radiation-grafted polyethylene-coated polypropylene filamentary fiber and its nonwoven cloth. Ligand contents and acid capacities of the resulting cation exchangers were ca. 2 mmol/g and ca. 4 mmol/g for the filamentary fiber and for the non-woven cloth, respectively. The selectivity sequence of nonwoven cloth shape for dialect metal ions is Mg(II)  Ca(II)
Ca(II)  Co(II)
Co(II)  Zn(II)
Zn(II)  Cd(II)
Cd(II)  Ni(II)
Ni(II)  Pb(II)
Pb(II)  Cu(II). Capacities in mmol/g at pH 5 were Ca(II) 0.91, Mg(II) 0.98, Cd(II) 1.5, Ni(II) 1.5, Pb(II) 1.6, Cu(II) 1.8. Column mode for filamentary fiber shape revealed that breakthrough capacities for Cu(II) (ca. 1 mmol/g) were not dependent on flow rates up to 200
Cu(II). Capacities in mmol/g at pH 5 were Ca(II) 0.91, Mg(II) 0.98, Cd(II) 1.5, Ni(II) 1.5, Pb(II) 1.6, Cu(II) 1.8. Column mode for filamentary fiber shape revealed that breakthrough capacities for Cu(II) (ca. 1 mmol/g) were not dependent on flow rates up to 200  300 h
300 h in space velocity.
in space velocity.
Seko, Noriaki; Katakai, Akio; Tamada, Masao; Sugo, Takanobu*; Yoshii, Fumio
Separation Science and Technology, 39(16), p.3753 - 3767, 2004/00
Times Cited Count:82 Percentile:89.98(Chemistry, Multidisciplinary)Fibrous amidoxime adsorbents were prepared by radiation-induced co-grafting of acrylonitrile (AN) and methacrylic acid (MAA) and subsequent amidoximation. Adsorption of uranium in seawater was evaluated by pumping seawater into the adsorbent column. The best monomer ratio of AN and MAA was 7:3 for continual usage of uranium adsorption. Though hydrochloric acid is an effective eluting agent for the metals adsorbed on the adsorbent, amidoxime groups were simultaneously damaged after five cycles of adsorption-desorption. This deterioration was reduced by an alkaline treatment of the adsorbents after each elution. Furthermore, various organic acids were examined as elution agents. It was found that the 80% of adsorption activity was still maintained after five cycles of adsorption-desorption when tartaric acid was used for eluting agent.
Seko, Noriaki; Katakai, Akio; Hasegawa, Shin; Tamada, Masao; Kasai, Noboru; Takeda, Hayato*; Sugo, Takanobu; Saito, Kyoichi*
Nuclear Technology, 144(2), p.274 - 278, 2003/11
Times Cited Count:127 Percentile:98.72(Nuclear Science & Technology)The total amount of uranium dissolved in seawater at a uniform concentration of 3 mg-U/m in the world's oceans is 4.5 billion tons. An adsorption method using polymeric adsorbents capable of specifically recovering uranium from seawater is reported to be economically feasible. A uranium-specific non-woven fabric was used as the adsorbent packed in an adsorption cage. We submerged adsorption cages, 16 m
in the world's oceans is 4.5 billion tons. An adsorption method using polymeric adsorbents capable of specifically recovering uranium from seawater is reported to be economically feasible. A uranium-specific non-woven fabric was used as the adsorbent packed in an adsorption cage. We submerged adsorption cages, 16 m in cross-sectional area and 16 cm in height, in the Pacific Ocean at a depth of 20 m at 7 km offshore of Japan. The cage consisted of stacks of 52,000 sheets of the uranium-specific non-woven fabric with a total mass of 350 kg. The total amount of uranium recovered by the non-woven fabric was more than one kg in terms of yellow cake during a total submersion time of 240 days in the ocean.
in cross-sectional area and 16 cm in height, in the Pacific Ocean at a depth of 20 m at 7 km offshore of Japan. The cage consisted of stacks of 52,000 sheets of the uranium-specific non-woven fabric with a total mass of 350 kg. The total amount of uranium recovered by the non-woven fabric was more than one kg in terms of yellow cake during a total submersion time of 240 days in the ocean.
Seko, Noriaki; Tamada, Masao; Sugo, Takanobu
Kaiyo Kaihatsu Nyusu, 31(1), p.8 - 11, 2003/04
no abstracts in English
Shiraishi, Tomoyuki*; Tamada, Masao; Saito, Kyoichi*; Sugo, Takanobu
Radiation Physics and Chemistry, 66(1), p.43 - 47, 2003/01
Times Cited Count:50 Percentile:93.91(Chemistry, Physical)no abstracts in English