Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Hayashizaki, Kohei; Hirooka, Shun; Yamada, Tadahisa*; Sunaoshi, Takeo*; Murakami, Tatsutoshi; Saito, Kosuke
Ceramics (Internet), 8(1), p.24_1 - 24_12, 2025/03
Vauchy, R.; Hirooka, Shun; Horii, Yuta; Ogasawara, Masahiro*; Sunaoshi, Takeo*; Yamada, Tadahisa*; Tamura, Tetsuya*; Murakami, Tatsutoshi
Journal of Nuclear Materials, 599, p.155233_1 - 155233_11, 2024/10
Times Cited Count:0 Percentile:0.00(Materials Science, Multidisciplinary)The fluorite exsolution/recombination in U Pu
Pu O
O (y = 0.30 and 0.45) and PuO
(y = 0.30 and 0.45) and PuO was investigated using differential scanning calorimetry. The results are in relatively good agreement with the literature data, except for plutonia. Our values indicate that the critical temperature of the miscibility gap in Pu-O is 30
was investigated using differential scanning calorimetry. The results are in relatively good agreement with the literature data, except for plutonia. Our values indicate that the critical temperature of the miscibility gap in Pu-O is 30 50 K lower than previously reported. Finally, the systematic experimental procedure allowed us refining the locus of the solvus existing in hypostoichiometric U
50 K lower than previously reported. Finally, the systematic experimental procedure allowed us refining the locus of the solvus existing in hypostoichiometric U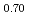 0Pu
0Pu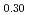 O
O , U
, U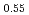 Pu
Pu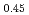 O
O , and PuO
, and PuO dioxides.
dioxides.
 Pu
Pu O
O powder using master sintering curve theory
powder using master sintering curve theoryNakamichi, Shinya; Sunaoshi, Takeo*; Hirooka, Shun; Vauchy, R.; Murakami, Tatsutoshi
Journal of Nuclear Materials, 595, p.155072_1 - 155072_11, 2024/07
Times Cited Count:1 Percentile:51.66(Materials Science, Multidisciplinary)Hirooka, Shun; Horii, Yuta; Sunaoshi, Takeo*; Uno, Hiroki*; Yamada, Tadahisa*; Vauchy, R.; Hayashizaki, Kohei; Nakamichi, Shinya; Murakami, Tatsutoshi; Kato, Masato
Journal of Nuclear Science and Technology, 60(11), p.1313 - 1323, 2023/11
Times Cited Count:5 Percentile:78.63(Nuclear Science & Technology)Additive MOX pellets are fabricated by a conventional dry powder metallurgy method. Nd O
O and Sm
and Sm O
O are chosen as the additive materials to simulate the corresponding soluble fission products dispersed in MOX. Shrinkage curves of the MOX pellets are obtained by dilatometry, which reveal that the sintering temperature is shifted toward a value higher than that of the respective regular MOX. The additives, however, promote grain growth and densification, which can be explained by the effect of oxidized uranium cations covering to a pentavalent state. Ceramography reveals large agglomerates after sintering, and Electron Probe Micro-Analysis confirms that inhomogeneous elemental distribution, whereas XRD reveals a single face-centered cubic phase. Finally, by grinding and re-sintering the specimens, the cation distribution homogeneity is significantly improved, which can simulate spent nuclear fuels with soluble fission products.
are chosen as the additive materials to simulate the corresponding soluble fission products dispersed in MOX. Shrinkage curves of the MOX pellets are obtained by dilatometry, which reveal that the sintering temperature is shifted toward a value higher than that of the respective regular MOX. The additives, however, promote grain growth and densification, which can be explained by the effect of oxidized uranium cations covering to a pentavalent state. Ceramography reveals large agglomerates after sintering, and Electron Probe Micro-Analysis confirms that inhomogeneous elemental distribution, whereas XRD reveals a single face-centered cubic phase. Finally, by grinding and re-sintering the specimens, the cation distribution homogeneity is significantly improved, which can simulate spent nuclear fuels with soluble fission products.
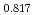 Pu
Pu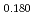 Am
Am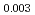 O
O uranium-plutonium-americium mixed oxides at 1573, 1773, and 1873 K
uranium-plutonium-americium mixed oxides at 1573, 1773, and 1873 KVauchy, R.; Sunaoshi, Takeo*; Hirooka, Shun; Nakamichi, Shinya; Murakami, Tatsutoshi; Kato, Masato
Journal of Nuclear Materials, 580, p.154416_1 - 154416_11, 2023/07
Times Cited Count:9 Percentile:93.25(Materials Science, Multidisciplinary) ceramics
ceramicsVauchy, R.; Hirooka, Shun; Watanabe, Masashi; Yokoyama, Keisuke; Sunaoshi, Takeo*; Yamada, Tadahisa*; Nakamichi, Shinya; Murakami, Tatsutoshi
Ceramics International, 49(2), p.3058 - 3065, 2023/01
Times Cited Count:11 Percentile:63.47(Materials Science, Ceramics) with Pu = 0.45 and 0.68 and related defect formation energy
with Pu = 0.45 and 0.68 and related defect formation energyHirooka, Shun; Matsumoto, Taku; Sunaoshi, Takeo*; Hino, Tetsushi*
Journal of Nuclear Materials, 558, p.153375_1 - 153375_8, 2022/01
Times Cited Count:5 Percentile:48.19(Materials Science, Multidisciplinary)no abstracts in English
 and (U,Pu,Am,Np)O
and (U,Pu,Am,Np)O
Hirooka, Shun; Matsumoto, Taku; Kato, Masato; Sunaoshi, Takeo*; Uno, Hiroki*; Yamada, Tadahisa*
Journal of Nuclear Materials, 542, p.152424_1 - 152424_9, 2020/12
Times Cited Count:12 Percentile:74.60(Materials Science, Multidisciplinary)The measurement of oxygen potential was conducted at 1,673, 1,773, and 1,873 K for (U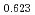 Pu
Pu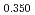 Am
Am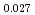 )O
)O and at 1,873 and 1,923 K for (U
and at 1,873 and 1,923 K for (U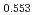 Pu
Pu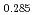 Am
Am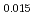 Np
Np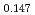 )O
)O by using a thermo-gravimeter and an oxygen sensor. Am inclusion in terms of substituting the U significantly increased the oxygen potential. Similarly, the inclusion of Np as a substitute for U increased the oxygen potential; however, the effect was not as large as that with the Pu or Am addition at the same rate. The results were analyzed via defect chemistry and certain defect formations were suggested in the reducing region and the near-stoichiometric region by plotting the relationship between PO
by using a thermo-gravimeter and an oxygen sensor. Am inclusion in terms of substituting the U significantly increased the oxygen potential. Similarly, the inclusion of Np as a substitute for U increased the oxygen potential; however, the effect was not as large as that with the Pu or Am addition at the same rate. The results were analyzed via defect chemistry and certain defect formations were suggested in the reducing region and the near-stoichiometric region by plotting the relationship between PO and the deviation from the stoichiometry. The equilibrium constants of the defect reactions were arranged to reproduce the experiment such that Am/Np contents were included in the entropy with coefficients fitting the experimental data.
and the deviation from the stoichiometry. The equilibrium constants of the defect reactions were arranged to reproduce the experiment such that Am/Np contents were included in the entropy with coefficients fitting the experimental data.
 at high temperatures of 1673-1873 K
at high temperatures of 1673-1873 KWatanabe, Masashi; Kato, Masato; Sunaoshi, Takeo*
Journal of Nuclear Materials, 542, p.152472_1 - 152472_7, 2020/12
Times Cited Count:3 Percentile:26.56(Materials Science, Multidisciplinary)The oxygen self-diffusion coefficients in near stoichiometric (U,Pu)O at high temperatures were successfully measured by thermogravimetry combined with the oxygen isotope exchange method. The activation energy for oxygen diffusion in the stoichiometric composition of (U,Pu)O
at high temperatures were successfully measured by thermogravimetry combined with the oxygen isotope exchange method. The activation energy for oxygen diffusion in the stoichiometric composition of (U,Pu)O was evaluated from experimental data, and the value was determined to be 248 kJ/mol. In addition, the defect migration energies of (U,Pu)O
was evaluated from experimental data, and the value was determined to be 248 kJ/mol. In addition, the defect migration energies of (U,Pu)O were derived, and the oxygen self-diffusion coefficients were evaluated using these. As a result, good agreement was found between the experimental data and the oxygen self-diffusion coefficients calculated using the defect migration energies.
were derived, and the oxygen self-diffusion coefficients were evaluated using these. As a result, good agreement was found between the experimental data and the oxygen self-diffusion coefficients calculated using the defect migration energies.
 U
U )O
)O
Nakamichi, Shinya; Hirooka, Shun; Kato, Masato; Sunaoshi, Takeo*; Nelson, A. T.*; McClellan, K. J.*
Journal of Nuclear Materials, 535, p.152188_1 - 152188_8, 2020/07
Times Cited Count:15 Percentile:81.56(Materials Science, Multidisciplinary)Oxygen-to-metal ratio (O/M) of uranium and plutonium mixed oxide depends on its oxygen partial pressure. To attain the desirable microstructure and O/M ratio of sintered pellets, it is important to investigate the relation between the sintering behavior and the atmosphere of sintering process. In this study, sintering behavior of (Pu U
U )O
)O and (Pu
and (Pu U
U )O
)O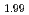 in controlled po
in controlled po atmosphere were investigated. It was found activation energy of (Pu
atmosphere were investigated. It was found activation energy of (Pu U
U )O
)O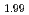 was higher than that of (Pu
was higher than that of (Pu U
U )O
)O . On the other hand, it was observed grain growth during sintering was suppressed in hypo-stoichiometric composition.
. On the other hand, it was observed grain growth during sintering was suppressed in hypo-stoichiometric composition.

Suzuki, Kiichi; Kato, Masato; Sunaoshi, Takeo*; Uno, Hiroki*; Carvajal-Nunez, U.*; Nelson, A. T.*; McClellan, K. J.*
Journal of the American Ceramic Society, 102(4), p.1994 - 2008, 2019/04
Times Cited Count:54 Percentile:93.21(Materials Science, Ceramics)The fundamental properties of CeO were assessed using a range of experimental techniques. The oxygen potential of CeO
were assessed using a range of experimental techniques. The oxygen potential of CeO was measured by the thermogravimetric technique, and a numerical fit for the oxygen potential of CeO
was measured by the thermogravimetric technique, and a numerical fit for the oxygen potential of CeO is derived based on defect chemistry. Mechanical properties of CeO
is derived based on defect chemistry. Mechanical properties of CeO were obtained using sound velocity measurement, resonant ultrasound spectroscopy and nanoindentation. The obtained mechanical properties of CeO
were obtained using sound velocity measurement, resonant ultrasound spectroscopy and nanoindentation. The obtained mechanical properties of CeO are then used to evaluate the Debye temperature and Gruneisen constant. The heat capacity and thermal conductivity of CeO
are then used to evaluate the Debye temperature and Gruneisen constant. The heat capacity and thermal conductivity of CeO were also calculated using the Debye temperature and the Gruneisen constant. Finally, the thermal conductivity was calculated based upon laser flash analysis measurements. This result demonstrates that the thermal conductivity has strong dependence upon material purity.
were also calculated using the Debye temperature and the Gruneisen constant. Finally, the thermal conductivity was calculated based upon laser flash analysis measurements. This result demonstrates that the thermal conductivity has strong dependence upon material purity.

Watanabe, Masashi; Sunaoshi, Takeo*; Kato, Masato
Defect and Diffusion Forum, 375, p.84 - 90, 2017/05
The oxygen chemical diffusion coefficient in (U, Pu)O was determined by thermo-gravimetry as functions of the Pu content, oxygen-to-metal ratio and temperature. The surface reaction was considered in the diffusion coefficient determination. The activation energy for the chemical diffusion coefficient was 60 kJ/mol and 65 kJ/mol, respectively, in (U
was determined by thermo-gravimetry as functions of the Pu content, oxygen-to-metal ratio and temperature. The surface reaction was considered in the diffusion coefficient determination. The activation energy for the chemical diffusion coefficient was 60 kJ/mol and 65 kJ/mol, respectively, in (U Pu
Pu )O
)O and (U
and (U Pu
Pu )O
)O .
.

Watanabe, Masashi; Kato, Masato; Sunaoshi, Takeo*
Transactions of the American Nuclear Society, 114, p.1081 - 1082, 2016/06
Many studies on the oxygen potential of UO have been carried out so far. However, the oxygen potential data for UO
have been carried out so far. However, the oxygen potential data for UO near the stoichiometric composition in the high temperature region (1673-1873 K) are limited. In this work, the oxygen potential data of UO
near the stoichiometric composition in the high temperature region (1673-1873 K) are limited. In this work, the oxygen potential data of UO were extended to high temperature range of 1673-1873 K by gas equilibrium method. The measured data were analyzed based on a defect chemistry model.
were extended to high temperature range of 1673-1873 K by gas equilibrium method. The measured data were analyzed based on a defect chemistry model.
 in oxygen partial pressure-controlled atmosphere
in oxygen partial pressure-controlled atmosphereKato, Masato; Ikusawa, Yoshihisa; Sunaoshi, Takeo*; Nelson, A. T.*; McClellan, K. J.*
Journal of Nuclear Materials, 469, p.223 - 227, 2016/02
Times Cited Count:14 Percentile:75.04(Materials Science, Multidisciplinary)Thermal expansion of (U Pu
Pu )O
)O (x = 0, 0.01, 0.02, 0.03) and (U
(x = 0, 0.01, 0.02, 0.03) and (U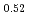 Pu
Pu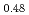 )O
)O was measured with a dilatometer in an oxygen partial pressure-controlled atmosphere. The oxygen partial pressure was controlled to hold a constant oxygen-to-metal ratio in the (U,Pu)O
was measured with a dilatometer in an oxygen partial pressure-controlled atmosphere. The oxygen partial pressure was controlled to hold a constant oxygen-to-metal ratio in the (U,Pu)O during the measurement. Thermal expansion slightly increased with the decrease in oxygen-to-metal ratio. The relationship was derived to describe thermal expansion.
during the measurement. Thermal expansion slightly increased with the decrease in oxygen-to-metal ratio. The relationship was derived to describe thermal expansion.
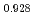 Am
Am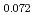 )O
)O at high temperatures
at high temperaturesMatsumoto, Taku; Arima, Tatsumi*; Inagaki, Yaohiro*; Idemitsu, Kazuya*; Kato, Masato; Morimoto, Kyoichi; Sunaoshi, Takeo*
Journal of Nuclear Science and Technology, 52(10), p.1296 - 1302, 2015/10
Times Cited Count:11 Percentile:63.64(Nuclear Science & Technology)The oxygen potentials of (Pu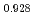 Am
Am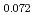 )O
)O were measured at 1873K, 1773K and 1473K by gas equilibrium method. It was shown that following the reduction of Am at the O/M ratio above 1.96, Pu was reduced at the O/M ratio below 1.96.
were measured at 1873K, 1773K and 1473K by gas equilibrium method. It was shown that following the reduction of Am at the O/M ratio above 1.96, Pu was reduced at the O/M ratio below 1.96.
 and (U,Pu)O
and (U,Pu)O
Nakamichi, Shinya; Hirooka, Shun; Sunaoshi, Takeo*; Kato, Masato; Nelson, A.*; McClellan, K.*
Transactions of the American Nuclear Society, 113(1), p.617 - 618, 2015/10
Cerium dioxide has been used as a surrogate material for plutonium dioxide. Dorr et al reported the use of hyper-stoichiometric conditions causes the start of shrinkage of (U,Ce)O at low temperature compared with the sintering in reducing atmosphere. However, the precise stoichiometry of the samples investigated was not controlled or otherwise monitored, preventing any quantitative conclusions regarding the similarities or differences between (U,Ce)O
at low temperature compared with the sintering in reducing atmosphere. However, the precise stoichiometry of the samples investigated was not controlled or otherwise monitored, preventing any quantitative conclusions regarding the similarities or differences between (U,Ce)O and (U,Pu)O
and (U,Pu)O . The motivation for the present work is therefore to compare the sintering behavior of MOX and the (U,Ce)O
. The motivation for the present work is therefore to compare the sintering behavior of MOX and the (U,Ce)O MOX surrogates under controlled atmospheres to assess the role of oxygen defects on densification in both systems.
MOX surrogates under controlled atmospheres to assess the role of oxygen defects on densification in both systems.

Uchida, Teppei; Sunaoshi, Takeo*; Konashi, Kenji*; Kato, Masato
Journal of Nuclear Materials, 452(1-3), p.281 - 284, 2014/09
Times Cited Count:12 Percentile:61.00(Materials Science, Multidisciplinary)Experiment and simulation studies on physical properties of actinide oxides have been carried out. Thermal expansion is important data to evaluate the various properties from molecular dynamics (MD) simulation. In this study, thermal expansion of PuO was evaluated by experiment and MD simulation. In the experimental study, thermal expansion of PuO
was evaluated by experiment and MD simulation. In the experimental study, thermal expansion of PuO pellet was determined by a dilatometer in the temperature range of 300-1923 K. In the MD simulation, Born-Mayer-Huggins interatomic potential with a partially ionic model and Morse potential were employed. Lattice constants of PuO
pellet was determined by a dilatometer in the temperature range of 300-1923 K. In the MD simulation, Born-Mayer-Huggins interatomic potential with a partially ionic model and Morse potential were employed. Lattice constants of PuO were evaluated in the temperature range of 300-2800 K by MD simulation, and thermal expansion was evaluated. The experimental data was good agreement with the MD simulation result. Evaluation formula for thermal expansion of PuO
were evaluated in the temperature range of 300-2800 K by MD simulation, and thermal expansion was evaluated. The experimental data was good agreement with the MD simulation result. Evaluation formula for thermal expansion of PuO in the temperature range of 300-2800 K was derived from both data.
in the temperature range of 300-2800 K was derived from both data.

Kato, Masato; Uchida, Teppei; Matsumoto, Taku; Sunaoshi, Takeo*; Nakamura, Hiroki; Machida, Masahiko
Journal of Nuclear Materials, 451(1-3), p.78 - 81, 2014/08
Times Cited Count:11 Percentile:61.00(Materials Science, Multidisciplinary)Linear thermal expansion of PuO was measured by dilatometry in an oxygen partial pressure-controlled atmosphere. The measured data of PuO
was measured by dilatometry in an oxygen partial pressure-controlled atmosphere. The measured data of PuO slightly increased with deviation
slightly increased with deviation  . The linear thermal expansion of PuO
. The linear thermal expansion of PuO was determined as a function of temperature and O/M ratio, and the equation for the thermal expansion coefficient was derived. Heat capacity of PuO
was determined as a function of temperature and O/M ratio, and the equation for the thermal expansion coefficient was derived. Heat capacity of PuO was evaluated using this equation. The effect of O/M ratio on heat capacity was small. In addition to the vibration and dilatational terms, it is important to analyze the Schottky term in evaluating heat capacity of PuO
was evaluated using this equation. The effect of O/M ratio on heat capacity was small. In addition to the vibration and dilatational terms, it is important to analyze the Schottky term in evaluating heat capacity of PuO .
.
 ,Pu
,Pu )O
)O in
in 
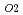 -controlled atmosphere
-controlled atmosphereKato, Masato; Murakami, Tatsutoshi; Sunaoshi, Takeo*; Nelson, A. T.*; McClellan, K.*
Proceedings of International Nuclear Fuel Cycle Conference; Nuclear Energy at a Crossroads (GLOBAL 2013) (CD-ROM), p.852 - 856, 2013/09
Uranium and plutonium mixed oxide (U ,Pu
,Pu )O
)O which has been developed as a fast reactor fuel is nonstoichiometric compound. The stoichiometry significantly affects various properties. The relationship between oxygen potential and oxygen-to-metal (O/M) ratio has been investigated so far. The measurement results showed that it is essential to control oxygen partial pressure (
which has been developed as a fast reactor fuel is nonstoichiometric compound. The stoichiometry significantly affects various properties. The relationship between oxygen potential and oxygen-to-metal (O/M) ratio has been investigated so far. The measurement results showed that it is essential to control oxygen partial pressure (
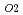 ) for holding constant O/M ratio in high temperature range. Therefore, properties of (U
) for holding constant O/M ratio in high temperature range. Therefore, properties of (U ,Pu
,Pu )O
)O were measured in
were measured in 
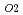 -controlled atmosphere in this work. In this work, properties of O/M change, sintering and thermal expansion were investigated in
-controlled atmosphere in this work. In this work, properties of O/M change, sintering and thermal expansion were investigated in 
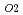 -controlled atmosphere.
-controlled atmosphere.
 and analysis of oxygen diffusion in PuO
and analysis of oxygen diffusion in PuO and (Pu,U)O
and (Pu,U)O
Kato, Masato; Uchida, Teppei; Sunaoshi, Takeo*
Physica Status Solidi (C), 10(2), p.189 - 192, 2013/02
Times Cited Count:6 Percentile:90.85(Materials Science, Multidisciplinary)