Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Dohi, Terumi; Iijima, Kazuki; Machida, Masahiko; Suno, Hiroya*; Omura, Yoshihito*; Fujiwara, Kenso; Kimura, Shigeru*; Kanno, Futoshi*
Environmental Radiochemical Analysis VII, p.50 - 57, 2023/12
 microscale localisation observation
microscale localisation observationDohi, Terumi; Iijima, Kazuki; Machida, Masahiko; Suno, Hiroya*; Omura, Yoshihito*; Fujiwara, Kenso; Kimura, Shigeru*; Kanno, Futoshi*
PLOS ONE (Internet), 17(7), p.e0271035_1 - e0271035_21, 2022/07
Times Cited Count:4 Percentile:26.49(Multidisciplinary Sciences)Suno, Hiroya; Machida, Masahiko; Dohi, Terumi; Omura, Yoshihito*
Isotope News, (781), p.8 - 12, 2022/06
no abstracts in English
Suno, Hiroya; Machida, Masahiko
ACS Food Science & Technology (Internet), 1(8), p.1381 - 1391, 2021/09
Generally, fungi accumulate radioactive Cs much more than higher plants. Particularly, some species of fungi have been well-known to selectively accumulate high amounts of radioactive Cs ( Cs,
Cs,  Cs) released by nuclear accidents in their fruiting bodies compared with other species. This is a highly concerned issue in the contaminated forest areas, because consumption of mushrooms is one of the main routes of internal radiation for the residents in addition to consumption of other contaminated forest foods. So far, several investigations have focused on only one pigment molecule, norbadione A, observed mainly inside the mushroom caps, selectively complexing Cs cations, though there exist a huge variety of pigment molecules inside mushrooms. Here, we examine systematically which type of pigment molecules can selectively complex Cs cations, by using a state-of-the-art computational technique. We consequently find that a symmetric scissors-like structure, formed with two equivalent pulvinic acid moieties, is crucial for the Cs complexation selectivity, from comparative analysis among four scissors-like pigments. We thus predict that mushroom species including such symmetric scissors-like pigments can keep high Cs radioactivity.
Cs) released by nuclear accidents in their fruiting bodies compared with other species. This is a highly concerned issue in the contaminated forest areas, because consumption of mushrooms is one of the main routes of internal radiation for the residents in addition to consumption of other contaminated forest foods. So far, several investigations have focused on only one pigment molecule, norbadione A, observed mainly inside the mushroom caps, selectively complexing Cs cations, though there exist a huge variety of pigment molecules inside mushrooms. Here, we examine systematically which type of pigment molecules can selectively complex Cs cations, by using a state-of-the-art computational technique. We consequently find that a symmetric scissors-like structure, formed with two equivalent pulvinic acid moieties, is crucial for the Cs complexation selectivity, from comparative analysis among four scissors-like pigments. We thus predict that mushroom species including such symmetric scissors-like pigments can keep high Cs radioactivity.
Suno, Hiroya; Machida, Masahiko; Dohi, Terumi; Omura, Yoshihito*
Scientific Reports (Internet), 11(1), p.8228_1 - 8228_13, 2021/04
Times Cited Count:6 Percentile:21.89(Multidisciplinary Sciences)We evaluate stability of caesium (Cs) and other alkali-metal cation complexes of lichen metabolites in both gas and aqueous phases to discuss why lichens can retain radioactive Cs in the thalli over several years. We focus on oxalic acid, (+)-usnic acid, atranorin, lecanoric acid, and protocetraric acid, which are common metabolite substances in various lichens including, e.g.,  and
and  retaining Cs in Fukushima, Japan. By performing quantum chemical calculations, their gas-phase complexation energies and aqueous-solution complexation free energies with alkali-metal cations are computed for their neutral and deprotonated cases. Consequently, all the molecules are found to energetically favor cation complexations and the preference order is Li
retaining Cs in Fukushima, Japan. By performing quantum chemical calculations, their gas-phase complexation energies and aqueous-solution complexation free energies with alkali-metal cations are computed for their neutral and deprotonated cases. Consequently, all the molecules are found to energetically favor cation complexations and the preference order is Li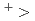 Na
Na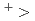 K
K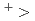 Rb
Rb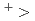 Cs
Cs for all conditions, indicating no specific Cs selectivity but strong binding with all alkali cations. Comparing complexation stabilities among these metabolites, lecanoric and protocetraric acids seen in medullary layer are found to keep higher affinity in their neutral case, while (+)-usnic acid and atranorin in upper cortex exhibit rather strong affinity only in deprotonated cases through forming stable six atoms' ring containing alkali cation chelated by two oxygens. These results suggest that the medullary layer can catch all alkali cations in a wide pH range around the physiological one, while the upper cortex can effectively block penetration of metal ions when the metal stress grows. Such insights highlight a physiological role of metabolites like blocking of metal-cation migrations into intracellular tissues, and explain long-term retention of alkali cations including Cs in lichens containing enough such metabolites to bind them.
for all conditions, indicating no specific Cs selectivity but strong binding with all alkali cations. Comparing complexation stabilities among these metabolites, lecanoric and protocetraric acids seen in medullary layer are found to keep higher affinity in their neutral case, while (+)-usnic acid and atranorin in upper cortex exhibit rather strong affinity only in deprotonated cases through forming stable six atoms' ring containing alkali cation chelated by two oxygens. These results suggest that the medullary layer can catch all alkali cations in a wide pH range around the physiological one, while the upper cortex can effectively block penetration of metal ions when the metal stress grows. Such insights highlight a physiological role of metabolites like blocking of metal-cation migrations into intracellular tissues, and explain long-term retention of alkali cations including Cs in lichens containing enough such metabolites to bind them.
Suno, Hiroya; Machida, Masahiko
RIST News, (66), p.3 - 16, 2020/10
no abstracts in English
Suno, Hiroya; Okumura, Masahiko; Machida, Masahiko
Jiban Kogakkai-Shi, 67(10), p.34 - 35, 2019/10
no abstracts in English
 , K
, K , and Na
, and Na in gas and aqueous phases
in gas and aqueous phasesSuno, Hiroya; Machida, Masahiko
Chemical Physics Letters, 730, p.26 - 31, 2019/09
Times Cited Count:1 Percentile:3.26(Chemistry, Physical)We perform quantum chemical calculations for the Cs , K
, K , and Na
, and Na complexes of norbadione A (NBA), a pigment molecule in mushrooms known to accumulate Cs
complexes of norbadione A (NBA), a pigment molecule in mushrooms known to accumulate Cs . A numerical two-step approach, by Ota
. A numerical two-step approach, by Ota 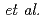 , is employed to examine its alkali-metal-cation complexation selectivity in aqueous solutions. Applying it to the neutral, di- and tetra-deprotonated NBAs, we confirm that the complexation selectivity on Cs
, is employed to examine its alkali-metal-cation complexation selectivity in aqueous solutions. Applying it to the neutral, di- and tetra-deprotonated NBAs, we confirm that the complexation selectivity on Cs emerges only in high pHs, in which the di-protonated NBA dominates, in agreement with experimental results. This is the first demonstration of the approach for a biological molecule whose selectivity is known to be anomalous.
emerges only in high pHs, in which the di-protonated NBA dominates, in agreement with experimental results. This is the first demonstration of the approach for a biological molecule whose selectivity is known to be anomalous.
Dohi, Terumi; Iijima, Kazuki; Machida, Masahiko; Suno, Hiroya*; Omura, Yoshihito*; Fujiwara, Kenso; Kimura, Shigeru*; Kanno, Futoshi*
no journal, ,
no abstracts in English
Suno, Hiroya; Machida, Masahiko; Dohi, Terumi; Omura, Yoshihito*; Sasaki, Yoshito
no journal, ,
Complexation of biomolecules with radiocesium is known to play an important role in the accumulation and migration of radioactivity on the organic matter in forests. In this work, we identify typical molecules forming a metal complex in the forest organic matter and carry out atomistic analyses by performing quantum mechanical calculations in order to understand the alkali metal cation complexation selectivity of these organic molecules. Among these molecules, of particular interest are norbadione A (C H
H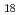 O
O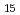 ), a primary pigment molecule present in mushrooms, oxalic acid (C
), a primary pigment molecule present in mushrooms, oxalic acid (C H
H O
O ), atranorin (C
), atranorin (C H
H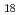 O
O ), lecanoric acid (C
), lecanoric acid (C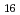 H
H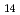 O
O ), and usnic acid (C
), and usnic acid (C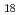 H
H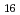 O
O ), main secondary metabolites in lichens.
), main secondary metabolites in lichens.
Dohi, Terumi; Iijima, Kazuki; Machida, Masahiko; Suno, Hiroya*; Omura, Yoshihito*; Fujiwara, Kenso; Kimura, Shigeru*; Kanno, Futoshi*
no journal, ,
no abstracts in English
Suno, Hiroya; Machida, Masahiko; Dohi, Terumi
no journal, ,
no abstracts in English