Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Rizaal, M.; Nakajima, Kunihisa; Suzuki, Eriko; Miwa, Shuhei
Annals of Nuclear Energy, 218, p.111433_1 - 111433_10, 2025/08
Times Cited Count:0 Percentile:0.00(Nuclear Science & Technology) reaction system
reaction systemRizaal, M.; Nakajima, Kunihisa; Suzuki, Eriko; Miwa, Shuhei
Proceedings of 11th European Review Meeting on Severe Accident Research Conference (ERMSAR 2024) (Internet), 11 Pages, 2024/05
Miwa, Shuhei; Karasawa, Hidetoshi; Nakajima, Kunihisa; Kino, Chiaki*; Suzuki, Eriko; Imoto, Jumpei
JAEA-Data/Code 2021-022, 32 Pages, 2023/01
The improved model for cesium (Cs) chemisorption onto stainless steel (SS) in the fission product (FP) chemistry database named ECUME was incorporated into the severe accident (SA) analysis code SAMPSON for the more accurate estimation of Cs distribution within nuclear reactor vessels in the TEPCO's Fukushima Daiichi Nuclear Power Station (1F). The SAMPSON with the improved model was verified based on the analysis results reproducing the experimental results which were subjected to the modeling of Cs chemisorption behavior. Then, the experiment in the facility with the temperature gradient tube to simulate SA conditions such as temperature decrease and aerosol formation was analyzed to confirm availability of the improved model to the analysis of Cs chemisorption onto SS. The SAMPSON with the improved model successfully reproduced the experimental results, which indicates that the improved model and the analytical method such as setting a method of node-junction, models of aerosol formation and the calculation method of saturated CsOH vapor pressure can be applicable to the analysis of Cs chemisorption behavior. As the information on water-solubility of Cs deposits was also prerequisite to estimate the Cs distribution in the 1F because Cs can be transported through aqueous phase after the SA, the water-solubility of chemisorbed Cs compounds was investigated. The chemisorbed compounds on SS304 have been identified to CsFeO at 873 K to 973 K with higher water-solubility, CsFeSiO
at 873 K to 973 K with higher water-solubility, CsFeSiO at 973 K to 1273 K and Cs
at 973 K to 1273 K and Cs Si
Si O
O at 1073 K to 1273 K with lower water-solubility. From these results, the water-solubility of chemisorbed Cs compounds can be estimated according to the SA analysis conditions such as temperature in the reactor and the CsOH concentration affecting the amount of chemisorbed Cs.
at 1073 K to 1273 K with lower water-solubility. From these results, the water-solubility of chemisorbed Cs compounds can be estimated according to the SA analysis conditions such as temperature in the reactor and the CsOH concentration affecting the amount of chemisorbed Cs.
Rizaal, M.; Miwa, Shuhei; Suzuki, Eriko; Imoto, Jumpei; Osaka, Masahiko; Gou llo, M.*
llo, M.*
ACS Omega (Internet), 6(48), p.32695 - 32708, 2021/12
Times Cited Count:5 Percentile:24.90(Chemistry, Multidisciplinary)Miwa, Shuhei; Miyahara, Naoya*; Nakajima, Kunihisa; Imoto, Jumpei; Suzuki, Eriko
Nihon Genshiryoku Gakkai-Shi ATOMO , 63(12), p.825 - 829, 2021/12
, 63(12), p.825 - 829, 2021/12
In the BWR severe accident, it was indicated that the control material boron significantly influences chemical behavior and transition behavior of cesium, which is important from the viewpoint of exposure, and it causes great uncertainty in the prediction of environmental release and distribution in the reactor. Therefore, in order to elucidate the important chemistry to be considered in severe accident analysis, we have developed the experimental setup that enables the evaluation of chemistry during transportation in the reactor, and evaluated cesium chemistry. Based on the results, we developed the chemistry database named ECUME composed of datasets and models that are the basis of chemical reaction analysis so that chemical behavior could be evaluated by severe accident analysis code.
Gou llo, M.*; Hokkinen, M.*; Suzuki, Eriko; Horiguchi, Naoki; Barrachin, M.*; Cousin, F.*
llo, M.*; Hokkinen, M.*; Suzuki, Eriko; Horiguchi, Naoki; Barrachin, M.*; Cousin, F.*
Progress in Nuclear Energy, 138, p.103818_1 - 103818_10, 2021/08
Times Cited Count:7 Percentile:61.36(Nuclear Science & Technology)The present work aimed to study the transport of caesium iodide particles through a Thermal Gradient Tube (TGT) from 1023 K to 453 K. Retention inside the tube was evaluated for laminar flowrates composed of argon and steam. Higher retention of particles was highlighted for the experiments using higher steam content and lower flowrate. The second phase of the experiment aimed at identifying the possible revaporization or/and resuspension processes after the deposition. Three atmosphere compositions (Ar/H O, Ar/H
O, Ar/H and Ar/Air) were investigated. The particles removed from what was deposited on the surface walls during the sampling phase exhibited a similar GMD in Ar/H
and Ar/Air) were investigated. The particles removed from what was deposited on the surface walls during the sampling phase exhibited a similar GMD in Ar/H O and Ar/H
O and Ar/H and a bigger diameter in Ar/Air. The experimental results were then analysed with the SOPHAEROS module of the ASTEC code. Overall, the results obtained during the first phase were in agreement with the measured experimental results and during second phase led to no resuspension process.
and a bigger diameter in Ar/Air. The experimental results were then analysed with the SOPHAEROS module of the ASTEC code. Overall, the results obtained during the first phase were in agreement with the measured experimental results and during second phase led to no resuspension process.
Miwa, Shuhei; Nakajima, Kunihisa; Suzuki, Chikashi; Rizaal, M.; Suzuki, Eriko; Horiguchi, Naoki; Osaka, Masahiko
Proceedings of Joint International Conference on Supercomputing in Nuclear Applications + Monte Carlo 2020 (SNA + MC 2020), p.253 - 260, 2020/12
We have proceeded the fundamental study for the improvement of the evaluation of fission product (FP) chemistry and the treatment of fine space resolution which are main issues for the evaluation of FP behaviors in a severe accident (SA). We have been developing FP chemistry database named ECUME for the improvement of SA analysis codes. We prepared thermodynamic data for Cs compounds which is no experimental data available by computational approach. Regarding the space resolution issue, we have been developing analysis tool named CHASER based on 3D-CFD code with the model for FP chemistry. More accurate evaluation of FP behavior can be achieved by incorporating ECUME to the CHASER.
 Si
Si O
O
Suzuki, Eriko; Nakajima, Kunihisa; Osaka, Masahiko; Oishi, Yuji*; Muta, Hiroaki*; Kurosaki, Ken*
Journal of Nuclear Science and Technology, 57(7), p.852 - 857, 2020/07
Times Cited Count:5 Percentile:42.20(Nuclear Science & Technology)The low temperature heat capacity of Cs Si
Si O
O , which is one of the cesium chemisorbed compounds onto stainless steel during severe accident of the light water nuclear reactor, was experimentally determined for the first time in the temperature range of 1.9 - 302 K. The experimentally determined heat capacity,
, which is one of the cesium chemisorbed compounds onto stainless steel during severe accident of the light water nuclear reactor, was experimentally determined for the first time in the temperature range of 1.9 - 302 K. The experimentally determined heat capacity, 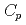
 (298.15K), and the standard entropy,
(298.15K), and the standard entropy, 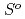 (298.15K), were 249.4
(298.15K), were 249.4  1.1 J K
1.1 J K mol
mol and 322.1
and 322.1  1.3 J K
1.3 J K mol
mol , respectively. The standard Gibbs energy of formation of Cs
, respectively. The standard Gibbs energy of formation of Cs Si
Si O
O at high temperatures,
at high temperatures, 

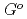 (
( ), were reevaluated by using the presently obtained
), were reevaluated by using the presently obtained 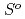 (298.15K) and the previously reported experimental results of the standard enthalpy of formation,
(298.15K) and the previously reported experimental results of the standard enthalpy of formation, 

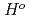 (298.15K), and the standard enthalpy increments at high temperatures,
(298.15K), and the standard enthalpy increments at high temperatures, 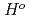 (
( )-
)-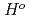 (298.15K).
(298.15K).
Miwa, Shuhei; Nakajima, Kunihisa; Miyahara, Naoya; Nishioka, Shunichiro; Suzuki, Eriko; Horiguchi, Naoki; Liu, J.; Miradji, F.; Imoto, Jumpei; Afiqa, B. M.; et al.
Mechanical Engineering Journal (Internet), 7(3), p.19-00537_1 - 19-00537_11, 2020/06
We constructed the fission product (FP) chemistry database named ECUME for LWR severe accident. This version of ECUME is equipped with dataset of the chemical reactions and their kinetics constants for the reactions of cesium(Cs)-iodine(I)-boron(B)-molybdenum(Mo)-oxygen(O)-hydrogen(H) system in gas phase, the elemental model for the high temperature chemical reaction of Cs with stainless steel applied as the structural material in a reactor, and thermodynamic data for CsBO vapor species and solids of Cs
vapor species and solids of Cs Si
Si O
O and CsFeSiO
and CsFeSiO for these chemical reactions. The ECUME will provide estimation of Cs distribution due to the evaluation of effects of interaction with BWR control material B and stainless steel on Cs behavior in the Fukushima Daiichi Nuclear Power Station.
for these chemical reactions. The ECUME will provide estimation of Cs distribution due to the evaluation of effects of interaction with BWR control material B and stainless steel on Cs behavior in the Fukushima Daiichi Nuclear Power Station.
Nakajima, Kunihisa; Nishioka, Shunichiro*; Suzuki, Eriko; Osaka, Masahiko
Mechanical Engineering Journal (Internet), 7(3), p.19-00564_1 - 19-00564_14, 2020/06
A large amount of cesium (Cs) chemisorbed onto stainless steel is predicted to be present especially in the upper region of reactor pressure vessel (RPV) during light water reactor severe accident (LWR SA) and a chemisorption model was developed for estimation of such amounts of Cs for stainless steel type 304 (SS304). However, this existing chemisorption model cannot accurately reproduce experimental results. Therefore, in this study, a modified Cs chemisorption model which accounts for silicon content in SS304 and concentration of cesium hydroxide (CsOH) in gaseous phases was constructed by combining penetration theory for gas-liquid mass transfer with chemical reaction and mass action law for CsOH decomposition at interface between gaseous and solid phases. As a result, it was found that the modified model was able to reproduce the experimental data more accurately than the existing model.
Nishioka, Shunichiro; Nakajima, Kunihisa; Suzuki, Eriko; Osaka, Masahiko
Journal of Nuclear Science and Technology, 56(11), p.988 - 995, 2019/11
Times Cited Count:14 Percentile:77.49(Nuclear Science & Technology)In order to contribute to improvement of Cs chemisorption model used in severe accident analysis codes, the influence of chemical factors (temperature, atmosphere, concentration of affecting chemical elements etc.) on the Cs chemisorption behaviour onto stainless steel was investigated experimentally. It was found that the surface reaction rate constant used in the current Cs-chemisorption model was influenced by not only temperature, as already known, but also atmosphere, cesium hydroxide (CsOH) concentration in the gas phase and silicon content in SS304. Such chemical factors should be considered for the construction of the improved Cs-chemisorption model. Another important finding is that the chemisorption behavior at lower temperatures, around 873 K, could differ from those above 1073 K. Namely, Cs-Fe-O compounds would form as the main Cs-chemisorbed compounds at 873 K while Cs-Si-Fe-O compounds at more than 1073 K.
Nakajima, Kunihisa; Nishioka, Shunichiro; Suzuki, Eriko; Osaka, Masahiko
Proceedings of 27th International Conference on Nuclear Engineering (ICONE-27) (Internet), 8 Pages, 2019/05
Cesium chemisorption models were developed for estimation of amount of cesium chemisorbed onto stainless steel type 304 (SS304) during light water reactor severe accident. However, existing chemisorption models cannot accurately reproduce experimental results. In this study, a modified cesium chemisorption model was constructed based on a penetration theory for gas-liquid mass transfer with chemical reaction and was able to adequately describe effects on concentration of cesium hydroxide in gaseous phase and silicon content in SS304. It was found that the modified model can more accurately reproduce the experimental data than the existing model.
Miwa, Shuhei; Miyahara, Naoya; Nakajima, Kunihisa; Nishioka, Shunichiro; Suzuki, Eriko; Horiguchi, Naoki; Liu, J.; Miradji, F.; Imoto, Jumpei; Afiqa, B. M.; et al.
Proceedings of 27th International Conference on Nuclear Engineering (ICONE-27) (Internet), 8 Pages, 2019/05
We constructed the first version of fission product (FP) chemistry database named ECUME for LWR severe accident. The first version of ECUME is equipped with dataset of the chemical reactions and their kinetics constants for the reactions of cesium(Cs)-iodine(I)-boron(B)-molybdenum(Mo)-oxygen(O)-hydrogen(H) system in gas phase, the elemental model for the high temperature chemical reaction of Cs with stainless steel, and thermodynamic data for CsBO vapor species and solids of Cs
vapor species and solids of Cs Si
Si O
O and CsFeSiO
and CsFeSiO . The ECUME will provide more accurate estimation of Cs distribution due to the evaluation of effects of interaction with BWR control material B and stainless steel on Cs behavior in the Fukushima Daiichi Nuclear Power Station.
. The ECUME will provide more accurate estimation of Cs distribution due to the evaluation of effects of interaction with BWR control material B and stainless steel on Cs behavior in the Fukushima Daiichi Nuclear Power Station.
Suzuki, Eriko; Takase, Gaku; Nakajima, Kunihisa; Nishioka, Shunichiro; Hashimoto, Naoyuki*; Isobe, Shigehito*; Osaka, Masahiko
Proceedings of International Topical Workshop on Fukushima Decommissioning Research (FDR 2019) (Internet), 4 Pages, 2019/05
In order to acquire the knowledge of the Cs chemisorption behaviour in the lower temperature region, the Cs chemisorbed compounds and the surface reaction rates were investigated by conducting the Cs chemisorption tests onto stainless steel at 873 and 973 K. As a result, The cesium ferrate compounds were revealed to be formed at this temperatures. It was seen that the dependences of surface reaction rate constant on this temperature were different from that at the higher temperature region. This behaviour leads to the conclusion that the Cs chemisorption model in the low temperature region should be newly constructed.
Mohamad, A.*; Nakajima, Kunihisa; Suzuki, Eriko; Miwa, Shuhei; Osaka, Masahiko; Oishi, Yuji*; Muta, Hiroaki*; Kurosaki, Ken*
Proceedings of International Topical Workshop on Fukushima Decommissioning Research (FDR 2019) (Internet), 4 Pages, 2019/05
In the accident of Fukushima Daiichi Nuclear Power Station, formation of a volatile SrCl could have occurred by the sea-water injection into the core. This can cause the release of non-volatile group Sr from the fuel to induce chemical reactions with reactor structural materials, such as stainless steel and Zircaloy (Zry) cladding. Such reactions could cause the changes in distribution of Sr in the reactor. Chemical reactions between Sr species and Zry were therefore investigated experimentally. As the result, it can be said that Sr vapor species were chemically trapped right after the release from fuel. This trapping effect of Sr by Zry-cladding implies a possibility of preferable Sr retention in the oxide phase of debris.
could have occurred by the sea-water injection into the core. This can cause the release of non-volatile group Sr from the fuel to induce chemical reactions with reactor structural materials, such as stainless steel and Zircaloy (Zry) cladding. Such reactions could cause the changes in distribution of Sr in the reactor. Chemical reactions between Sr species and Zry were therefore investigated experimentally. As the result, it can be said that Sr vapor species were chemically trapped right after the release from fuel. This trapping effect of Sr by Zry-cladding implies a possibility of preferable Sr retention in the oxide phase of debris.
Miradji, F.; Suzuki, Chikashi; Nishioka, Shunichiro; Suzuki, Eriko; Nakajima, Kunihisa; Osaka, Masahiko; Barrachin, M.*; Do, T. M. D.*; Murakami, Kenta*; Suzuki, Masahide*
Proceedings of 9th Conference on Severe Accident Research (ERMSAR 2019) (Internet), 21 Pages, 2019/03
 -based simulated fuel containing CsI
-based simulated fuel containing CsITakamatsu, Yuki*; Ishii, Hiroto*; Oishi, Yuji*; Muta, Hiroaki*; Yamanaka, Shinsuke*; Suzuki, Eriko; Nakajima, Kunihisa; Miwa, Shuhei; Osaka, Masahiko; Kurosaki, Ken*
Nihon Genshiryoku Gakkai Wabun Rombunshi, 17(3/4), p.106 - 110, 2018/12
In order to establish the synthesis method of simulated fuel contacting Cesium (Cs) which is required for the evaluation of physical/chemical characteristics in fuel and release behavior of Cs, sintering tests of the cerium dioxide (CeO ) based simulated fuels containing Cesium iodide (CsI) are performed by using spark plasma sintering (SPS) method. The sintered CeO
) based simulated fuels containing Cesium iodide (CsI) are performed by using spark plasma sintering (SPS) method. The sintered CeO pellets with homogeneous distribution of several micro meter of CsI spherical precipitates were successfully obtained by optimizing SPS conditions.
pellets with homogeneous distribution of several micro meter of CsI spherical precipitates were successfully obtained by optimizing SPS conditions.
Nakajima, Kunihisa; Suzuki, Eriko; Miyahara, Naoya; Osaka, Masahiko
Progress in Nuclear Science and Technology (Internet), 5, p.168 - 170, 2018/11
Chemical interaction between cesium (Cs) vapor and stainless steel (SS) surface known as chemisorption can cause a significant amount of Cs retention on the inner surfaces within the reactor pressure vessel during a light water reactor severe accident (SA). Although the chemisorption is known to be influenced with temperature and atmosphere, their dependancies are not yet fully understood. Therefore, the Cs chemisorption behaviours under mixed gases of steam and hydrogen were experimentally examined to contribute to a better understanding of the chemisorption behaviours under various atmospheres experienced during the SA at the Fukushima Daiichi Nuclear Power Station. As a result, it was found that the deposited amounts of Cs onto the SS in the steam-containing atmosphere were much higher than those in the no steam atmosphere. It was considered that Cs revaporization from a chemisorbed product was one of the reasons why the deposited amounts under the reducing atmosphere decreased.

Suzuki, Eriko; Nakajima, Kunihisa; Osaka, Masahiko
Progress in Nuclear Science and Technology (Internet), 5, p.165 - 167, 2018/11
In severe accident condition, CsFeSiO could be formed by Cesium (Cs) chemisorption onto reactor structural materials. For evaluation of re-vaporization behavior, effect of atmosphere on the vaporization behavior of CsFeSiO
could be formed by Cesium (Cs) chemisorption onto reactor structural materials. For evaluation of re-vaporization behavior, effect of atmosphere on the vaporization behavior of CsFeSiO at high temperature was investigated by thermal gravimetric-differential thermal analyzer (TG-DTA) experiments. As a result, it was found that vaporization of CsFeSiO
at high temperature was investigated by thermal gravimetric-differential thermal analyzer (TG-DTA) experiments. As a result, it was found that vaporization of CsFeSiO in reducing atmosphere (Ar-5%H
in reducing atmosphere (Ar-5%H ) started at relatively low temperature, about 800
) started at relatively low temperature, about 800 C, compared with in atmosphere containing H
C, compared with in atmosphere containing H O (Ar-5%H
O (Ar-5%H -5%H
-5%H O). It was inferred that a possible chemical reaction for the weight loss at around 800
O). It was inferred that a possible chemical reaction for the weight loss at around 800 C would occurred by the decomposition of CsFeSiO
C would occurred by the decomposition of CsFeSiO into volatile Cs vapor species under H
into volatile Cs vapor species under H .
.
Kobata, Masaaki; Okane, Tetsuo; Nakajima, Kunihisa; Suzuki, Eriko; Owada, Kenji; Kobayashi, Keisuke*; Yamagami, Hiroshi; Osaka, Masahiko
Journal of Nuclear Materials, 498, p.387 - 394, 2018/01
Times Cited Count:19 Percentile:84.57(Materials Science, Multidisciplinary)In this study, for the understandings of Cesium (Cs) adsorption behavior on structure materials in severe accidents at a light water nuclear reactor, the chemical state of Cs and its distribution on the surface of SUS304 stainless steel (SS) with different Si concentration were investigated by hard X-ray photoelectron spectroscopy (HAXPES) and scanning electron microscope / energy dispersive X-ray spectroscopy (SEM/EDX). As a result, it was found that Cs is selectively adsorbed at the site where Si distributes with high concentration. CsFeSiO is a dominant Cs products in the case of low Si content, mainly formed, while Cs
is a dominant Cs products in the case of low Si content, mainly formed, while Cs Si
Si O
O and Cs
and Cs Si
Si O
O are formed in addition to CsFeSiO
are formed in addition to CsFeSiO in the case of high Si content. The chemical forms of the Cs compounds produced in the adsorption process on the SS surface has a close correlation with the concentration and chemical states of Si originally included in SS.
in the case of high Si content. The chemical forms of the Cs compounds produced in the adsorption process on the SS surface has a close correlation with the concentration and chemical states of Si originally included in SS.