Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Narita, Hirokazu*; Maeda, Motoki*; Tokoro, Chiharu*; Suzuki, Tomoya*; Tanaka, Mikiya*; Shiwaku, Hideaki; Yaita, Tsuyoshi
RSC Advances (Internet), 13(25), p.17001 - 17007, 2023/06
Times Cited Count:1 Percentile:51.1(Chemistry, Multidisciplinary)no abstracts in English
Suzuki, Tomoya*; Otsubo, Ukyo*; Ogata, Takeshi*; Shiwaku, Hideaki; Kobayashi, Toru; Yaita, Tsuyoshi; Matsuoka, Mitsuaki*; Murayama, Norihiro*; Narita, Hirokazu*
Separation and Purification Technology, 308, p.122943_1 - 122943_7, 2023/03
Times Cited Count:2 Percentile:24.43(Engineering, Chemical)HNO leaching is used in recycling Pd metal from spent products that primarily contain Ag, and most Pd residues are separated from solutions containing Ag(I). However, a small amount of Pd(II) often remains in these Ag(I) solutions. Therefore, the separation of Pd(II) and Ag(I) in HNO
leaching is used in recycling Pd metal from spent products that primarily contain Ag, and most Pd residues are separated from solutions containing Ag(I). However, a small amount of Pd(II) often remains in these Ag(I) solutions. Therefore, the separation of Pd(II) and Ag(I) in HNO solutions is essential to promote efficient Pd recycling. In this study, the separation of Pd(II) and Ag(I) in HNO
solutions is essential to promote efficient Pd recycling. In this study, the separation of Pd(II) and Ag(I) in HNO solutions was investigated using four N-donor-type adsorbents functionalized with amine (R-Amine), iminodiacetic acid (R-IDA), pyridine (R-Py), or bis-picolylamine (R-BPA). R-Amine, R-IDA, and R-Py selectively adsorbed Pd(II) over Ag(I), Cu(II), Ni(II), and Fe(III) from HNO
solutions was investigated using four N-donor-type adsorbents functionalized with amine (R-Amine), iminodiacetic acid (R-IDA), pyridine (R-Py), or bis-picolylamine (R-BPA). R-Amine, R-IDA, and R-Py selectively adsorbed Pd(II) over Ag(I), Cu(II), Ni(II), and Fe(III) from HNO solutions (0.3-7 M), but R-Amine exhibited a lower Pd adsorption efficiency. In contrast,
solutions (0.3-7 M), but R-Amine exhibited a lower Pd adsorption efficiency. In contrast,  90% of Pd(II), Ag(I), and Cu(II) were adsorbed by R-BPA over the entire range of HNO
90% of Pd(II), Ag(I), and Cu(II) were adsorbed by R-BPA over the entire range of HNO concentrations. Structural analyses of the adsorbed metal ions using Fourier transform infrared spectroscopy and extended X-ray absorption fine structure spectroscopy revealed the separation mechanisms of the N-donor-type adsorbents. Pd(II) adsorption on R-IDA, R-Py, and R-BPA occurred via Pd(II) coordination of the functional groups (iminodiacetic acid, pyridine, and bis-picolylamine, respectively), whereas that on R-Amine occurred via anion exchange of NO
concentrations. Structural analyses of the adsorbed metal ions using Fourier transform infrared spectroscopy and extended X-ray absorption fine structure spectroscopy revealed the separation mechanisms of the N-donor-type adsorbents. Pd(II) adsorption on R-IDA, R-Py, and R-BPA occurred via Pd(II) coordination of the functional groups (iminodiacetic acid, pyridine, and bis-picolylamine, respectively), whereas that on R-Amine occurred via anion exchange of NO
 with [Pd(NO
with [Pd(NO )
) ]
] . The coordinative adsorption mechanisms resulted in the higher Pd(II) adsorption behaviors of R-IDA, R-Py, and R-BPA. HCl (5.0 M) and thiourea (0.1 M) eluents desorbed 83% of Pd(II) from R-IDA and 95% from R-Py, respectively. R-Py was the most effective Pd(II) adsorbent based on adsorption selectivity and desorption efficiency.
. The coordinative adsorption mechanisms resulted in the higher Pd(II) adsorption behaviors of R-IDA, R-Py, and R-BPA. HCl (5.0 M) and thiourea (0.1 M) eluents desorbed 83% of Pd(II) from R-IDA and 95% from R-Py, respectively. R-Py was the most effective Pd(II) adsorbent based on adsorption selectivity and desorption efficiency.
Suzuki, Tomoya*; Otsubo, Ukyo*; Ogata, Takeshi*; Shiwaku, Hideaki; Kobayashi, Toru; Yaita, Tsuyoshi; Matsuoka, Mitsuaki*; Murayama, Norihiro*; Narita, Hirokazu*
Dalton Transactions (Internet), 50(33), p.11390 - 11397, 2021/09
Times Cited Count:2 Percentile:21.56(Chemistry, Inorganic & Nuclear)no abstracts in English
 Mo-adsorption/
Mo-adsorption/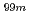 Tc-elution properties of alumina with different surface structures
Tc-elution properties of alumina with different surface structuresFujita, Yoshitaka; Seki, Misaki; Sano, Tadafumi*; Fujihara, Yasuyuki*; Kitagawa, Tomoya*; Matsukura, Minoru*; Hori, Junichi*; Suzuki, Tatsuya*; Tsuchiya, Kunihiko
Journal of Radioanalytical and Nuclear Chemistry, 327(3), p.1355 - 1363, 2021/03
Times Cited Count:3 Percentile:45.99(Chemistry, Analytical)We prepared three types of Al O
O with different surface structures and investigated
with different surface structures and investigated  Mo-adsorption/
Mo-adsorption/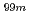 Tc-elution properties using [
Tc-elution properties using [ Mo]MoO
Mo]MoO that was irradiated in the Kyoto University Research Reactor. Al
that was irradiated in the Kyoto University Research Reactor. Al O
O adsorbed [
adsorbed [ Mo]molybdate ions in solutions at different pH; the lower was the pH, the higher was the Mo-adsorption capacity of Al
Mo]molybdate ions in solutions at different pH; the lower was the pH, the higher was the Mo-adsorption capacity of Al O
O . The
. The 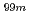 Tc-elution properties of molybdate ion adsorbed Al
Tc-elution properties of molybdate ion adsorbed Al O
O were elucidated by flowing saline. Consequently, it was suggested that
were elucidated by flowing saline. Consequently, it was suggested that  Mo-adsorption/desorption properties are affected by the specific surface of Al
Mo-adsorption/desorption properties are affected by the specific surface of Al O
O and
and 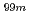 Tc-elution properties are affected by the crystal structure of Al
Tc-elution properties are affected by the crystal structure of Al O
O .
.
Nagae, Daisuke*; Abe, Yasushi*; Okada, Shunsuke*; Omika, Shuichiro*; Wakayama, Kiyoshi*; Hosoi, Shun*; Suzuki, Shinji*; Moriguchi, Tetsuro*; Amano, Masamichi*; Kamioka, Daiki*; et al.
Nuclear Instruments and Methods in Physics Research A, 986, p.164713_1 - 164713_7, 2021/01
Times Cited Count:5 Percentile:65.59(Instruments & Instrumentation)Narita, Hirokazu*; Kasuya, Ryo*; Suzuki, Tomoya*; Motokawa, Ryuhei; Tanaka, Mikiya*
Encyclopedia of Inorganic and Bioinorganic Chemistry (Internet), 28 Pages, 2020/12
Suzuki, Tomoya*; Ogata, Takeshi*; Tanaka, Mikiya*; Kobayashi, Toru; Shiwaku, Hideaki; Yaita, Tsuyoshi; Narita, Hirokazu*
Analytical Sciences, 35(12), p.1353 - 1360, 2019/12
Times Cited Count:3 Percentile:12.45(Chemistry, Analytical)no abstracts in English
Hayakawa, Sho*; Okita, Taira*; Itakura, Mitsuhiro; Kawabata, Tomoya*; Suzuki, Katsuyuki*
Journal of Materials Science, 54(16), p.11096 - 11110, 2019/08
Times Cited Count:11 Percentile:42.21(Materials Science, Multidisciplinary)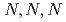 -trimethylglycine
-trimethylglycineSuzuki, Tomoya*; Narita, Hirokazu*; Ogata, Takeshi*; Suzuki, Hideya; Matsumura, Tatsuro; Kobayashi, Toru; Shiwaku, Hideaki; Yaita, Tsuyoshi
Solvent Extraction Research and Development, Japan, 26(1), p.11 - 19, 2019/06
Times Cited Count:3 Percentile:14.02(Chemistry, Multidisciplinary)The ability of AMP03, a styrene-divinylbenzene copolymer functionalized with 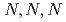 -trimethylglycine moieties, to adsorb Pd(II) from HNO
-trimethylglycine moieties, to adsorb Pd(II) from HNO solutions was investigated to elucidate the affinity of
solutions was investigated to elucidate the affinity of 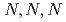 -trimethylglycine for Pd(II). In the present study, we investigated the mechanism of Pd(II) adsorption by AMP03 by means of adsorption experiments, Fourier Transform Infrared (FT-IR) spectroscopy, and Extended X-ray Absorption Fine Structure (EXAFS) spectroscopy.
-trimethylglycine for Pd(II). In the present study, we investigated the mechanism of Pd(II) adsorption by AMP03 by means of adsorption experiments, Fourier Transform Infrared (FT-IR) spectroscopy, and Extended X-ray Absorption Fine Structure (EXAFS) spectroscopy.
Nakanishi, Daiki*; Kawabata, Tomoya*; Doihara, Kohei*; Okita, Taira*; Itakura, Mitsuhiro; Suzuki, Katsuyuki*
Philosophical Magazine, 98(33), p.3034 - 3047, 2018/09
Times Cited Count:10 Percentile:47.84(Materials Science, Multidisciplinary)By using the six sets of interatomic potentials for face-centredcubic metals that differ in the stacking fault energy (SFE) while most of the other material parameters are kept almost identical, we conducted molecular dynamics simulations to evaluate the effects of SFE on the defect formation process through collision cascades. The ratio of glissile SIA clusters tends to decrease with increasing SFE. This is because perfect loops, the edges of which split into two partial dislocations with stacking fault structures between them in most cases, prefer to form at lower SFEs. The enhanced formation of glissile SIA clusters at lower SFEs can also be observed even at increased temperature.
Suzuki, Tomoya*; Ogata, Takeshi*; Tanaka, Mikiya*; Kobayashi, Toru; Shiwaku, Hideaki; Yaita, Tsuyoshi; Narita, Hirokazu*
Metals, 8(7), p.558_1 - 558_10, 2018/07
Times Cited Count:12 Percentile:53.94(Materials Science, Multidisciplinary)The refining of platinum group metals is based mainly on solvent extraction methods, whereas Ru is selectively recovered by distillation as RuO . Replacement of distillation byextraction is expected to simplify the purification process. To develop an effective extraction system for Ru, we analyzed the Ru species in HCl with UV-Vis and EXAFS spectroscopies, and we examined the properties of Ru extracted with N-2-ethylhexyl-bis(N-di-2-ethylhexyl-ethylamide) amine (EHBAA). EXAFS and UV-Vis spectra of Ru in HCl solutions revealed that the predominant Ru species in 0.5-10 M HCl solutions changed from [RuCl
. Replacement of distillation byextraction is expected to simplify the purification process. To develop an effective extraction system for Ru, we analyzed the Ru species in HCl with UV-Vis and EXAFS spectroscopies, and we examined the properties of Ru extracted with N-2-ethylhexyl-bis(N-di-2-ethylhexyl-ethylamide) amine (EHBAA). EXAFS and UV-Vis spectra of Ru in HCl solutions revealed that the predominant Ru species in 0.5-10 M HCl solutions changed from [RuCl (H
(H O)
O) ]
] to [RuCl
to [RuCl ]
]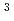
 with the HCl concentration. The extraction percentages of Ru in the EHBAA system increased with increasing HCl concentration, reached 80% at [HCl] = 5 M, and decreased athigher HCl concentrations. EXAFS analysis of the extracted complex indicated that the Ru
with the HCl concentration. The extraction percentages of Ru in the EHBAA system increased with increasing HCl concentration, reached 80% at [HCl] = 5 M, and decreased athigher HCl concentrations. EXAFS analysis of the extracted complex indicated that the Ru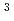
 had 5 Cl
had 5 Cl and 1 H
and 1 H O in its inner coordination sphere. The similarity of the dependence on HCl concentrations of the extraction in the EHBAA system and the distribution profile of [RuCl
O in its inner coordination sphere. The similarity of the dependence on HCl concentrations of the extraction in the EHBAA system and the distribution profile of [RuCl (H
(H O)]
O)]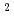
 on [RuCl
on [RuCl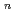 (H
(H O)
O)
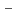
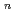 ]
]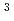

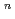 suggested that the EHBAA extracted the pentachlorido species.
suggested that the EHBAA extracted the pentachlorido species.
 Mo/
Mo/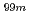 Tc generators
Tc generatorsSuzuki, Yoshitaka; Kitagawa, Tomoya*; Namekawa, Yoji*; Matsukura, Minoru*; Nishikata, Kaori; Mimura, Hitoshi*; Tsuchiya, Kunihiko
Transactions of the Materials Research Society of Japan, 43(2), p.75 - 80, 2018/04
no abstracts in English
Narita, Hirokazu*; Maeda, Motoki*; Tokoro, Chiharu*; Suzuki, Tomoya*; Tanaka, Mikiya*; Motokawa, Ryuhei; Shiwaku, Hideaki; Yaita, Tsuyoshi
Analytical Sciences, 33(11), p.1305 - 1309, 2017/11
Times Cited Count:11 Percentile:40.7(Chemistry, Analytical)Narita, Hirokazu*; Suzuki, Tomoya*; Motokawa, Ryuhei
Nihon Kinzoku Gakkai-Shi, 81(4), p.157 - 167, 2017/04
Times Cited Count:17 Percentile:62.95(Metallurgy & Metallurgical Engineering)Suzuki, Tomoya; Morita, Keisuke; Sasaki, Yuji; Matsumura, Tatsuro
Separation Science and Technology, 51(17), p.2815 - 2822, 2016/09
Times Cited Count:7 Percentile:23.23(Chemistry, Multidisciplinary)To understand the adsorption properties of styrene-divinylbenzene copolymer functionalized with 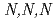 -trimethylglycine, AMP03, the adsorption behaviors for platinoid ions (Ru(III), Rh(III), and Pd(II)) were examined. Furthermore, we performed adsorption experiments using sample solutions with adding triethylamine, thiourea, and
-trimethylglycine, AMP03, the adsorption behaviors for platinoid ions (Ru(III), Rh(III), and Pd(II)) were examined. Furthermore, we performed adsorption experiments using sample solutions with adding triethylamine, thiourea, and 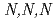 -trimethylglycine. Based on the adsorption data obtained in this study, we performed chromatographic experiments. The results indicated that all platinoid ions in the feed solution completely adsorbed on AMP03, and almost 80% of the adsorbed platinoid ions were recovered. These results show that AMP03 has the potential to recover Ru(III), Rh(III), and Pd(II) from high-level liquid waste.
-trimethylglycine. Based on the adsorption data obtained in this study, we performed chromatographic experiments. The results indicated that all platinoid ions in the feed solution completely adsorbed on AMP03, and almost 80% of the adsorbed platinoid ions were recovered. These results show that AMP03 has the potential to recover Ru(III), Rh(III), and Pd(II) from high-level liquid waste.
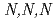 -trimethylglycine
-trimethylglycineSuzuki, Tomoya; Morita, Keisuke; Sasaki, Yuji; Matsumura, Tatsuro
Bulletin of the Chemical Society of Japan, 89(5), p.608 - 616, 2016/05
Times Cited Count:5 Percentile:16.1(Chemistry, Multidisciplinary)In a previous study, we found that AMP03 adsorbs Rh(III) from the HNO solution with pH = 1.7 - 2.2. In this study, to understand the Rh(III) adsorption properties of AMP03 and clarify conditions for efficient recovery, we investigated the adsorption behaviors of Rh(III) from the HNO
solution with pH = 1.7 - 2.2. In this study, to understand the Rh(III) adsorption properties of AMP03 and clarify conditions for efficient recovery, we investigated the adsorption behaviors of Rh(III) from the HNO solution. As a result, it was found that Rh(III) is effectively adsorbed from aqueous solution containing low H
solution. As a result, it was found that Rh(III) is effectively adsorbed from aqueous solution containing low H and high NO
and high NO
 concentration. Considering the adsorption mechanism of Rh(III), we found that Rh
concentration. Considering the adsorption mechanism of Rh(III), we found that Rh in aqueous solution is adsorbed with two betaine groups in AMP03 and three NO
in aqueous solution is adsorbed with two betaine groups in AMP03 and three NO
 .
.
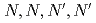 -tetraoctyl-thiodiglycolamide
-tetraoctyl-thiodiglycolamideSasaki, Yuji; Suzuki, Tomoya; Morita, Keisuke; Yoshizuka, Kazuharu*
Hydrometallurgy, 159, p.107 - 109, 2016/01
Times Cited Count:5 Percentile:27.46(Metallurgy & Metallurgical Engineering)The novel tridentate extractant including soft donor has developed and examined. The extractant, tetraoctyl-thiodiglycolamide (S-DGA), is analogous structure to tetraoctyl-diglycolamide (TODGA), but with sulfur donor instead of ether oxygen of TODGA. S-DGA has unique property to extract silver from acidic solutions to n-dodecane with relatively high D values. We investigated the extraction behavior of Ag in various acids, HNO , H
, H SO
SO , and HClO
, and HClO .
.
Sasaki, Yuji; Tsubata, Yasuhiro; Shirasu, Noriko; Morita, Keisuke; Suzuki, Tomoya
Nihon Genshiryoku Gakkai Wabun Rombunshi, 14(3), p.202 - 212, 2015/09
We have been developing the new partitioning method of high-level radioactive waste by single-cycle extraction process. This process is composed of extraction of actinides (An) and fission products (FP, e.g., Pd, Ru, Mo and Tc), and mutual separation by back-extraction. The extractant employed in this process is required to extract soft, hard acid metals and oxonium anions simultaneously. The NTAamide (hexaoctyl-nitrilotriacetamide) is one of the candidate extractants. After extraction of An and FP, the mutual separation by back-extraction should be set up. Pd and Ru extracted by NTAamide can be back-extracted by complexing agents such as thiourea, systeine, diethylenetriamine, and trisaminoethylamine, and the back-extraction of Mo can be performed by methylimino-diethylacetamide (MIDEA), NTAamide(C2) and iminodimethylphosphoric acid, and Re can be done by aqueous phase with high pH.
Sasaki, Yuji; Tsubata, Yasuhiro; Shirasu, Noriko; Morita, Keisuke; Suzuki, Tomoya
Proceedings of 21st International Conference & Exhibition; Nuclear Fuel Cycle for a Low-Carbon Future (GLOBAL 2015) (USB Flash Drive), p.1653 - 1656, 2015/09
The concept for the new partitioning method of high-level radioactive waste (HLW) by single-cycle extraction process has been investigated. This process is based on extraction of actinides (An) and fission products (FP), and mutual separation by reverse extraction. Solo extractant and several stripping reagents will be utilized in this process. The extractant employed in this process is required to extract soft (platinum metals), hard acid metals(An), and oxonium anions (Mo, Tc) simultaneously. NTAamide is one of the candidate extractants. After extraction of An and FP by NTAamide(C8), the mutual separation among these metals by reverse extraction will be followed using the suitable water-miscible reagents. The extraction of An and FP, and the masking effect by some water-miscible reagents has been studied.
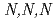 -trimethylglycine to Ru(III), Rh(III) and Pd(II) for developing separation techniques from high-level liquid waste
-trimethylglycine to Ru(III), Rh(III) and Pd(II) for developing separation techniques from high-level liquid wasteSuzuki, Tomoya; Shimazaki, Shoma*; Morita, Keisuke; Sasaki, Yuji; Ozawa, Masaki*; Matsumura, Tatsuro
Proceedings of 21st International Conference & Exhibition; Nuclear Fuel Cycle for a Low-Carbon Future (GLOBAL 2015) (USB Flash Drive), p.1539 - 1543, 2015/09
To understand the adsorption behaviors of ion-exchange resin bearing 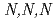 -trimethylglycine groups (AMP03), adsorption experiments using HNO
-trimethylglycine groups (AMP03), adsorption experiments using HNO solutions containing Ru(III), Rh(III), and Pd(II) have been performed. AMP03 strongly adsorbed Pd(II), and Ru(III) and Rh(III) were moderately adsorbed. To control the adsorption abilities of AMP03 for the Ru(III), Rh(III) and Pd(II), adsorption experiments using sample solutions with added thiourea (TU), triethylamine (TEA), and betaine anhydrous were performed. The adsorption abilities for Ru(III) and Rh(III) greatly increased with the addition of TEA, whereas the ability to adsorb Pd(II) was not significantly affected. The addition of TU or betaine anhydrous remarkably decreased the adsorption ability for Pd(II), and contrastingly slight changes in the abilities to adsorb Ru and Rh were also observed. These results show that AMP03 has a significant potential for separation of platinoid elements from HNO
solutions containing Ru(III), Rh(III), and Pd(II) have been performed. AMP03 strongly adsorbed Pd(II), and Ru(III) and Rh(III) were moderately adsorbed. To control the adsorption abilities of AMP03 for the Ru(III), Rh(III) and Pd(II), adsorption experiments using sample solutions with added thiourea (TU), triethylamine (TEA), and betaine anhydrous were performed. The adsorption abilities for Ru(III) and Rh(III) greatly increased with the addition of TEA, whereas the ability to adsorb Pd(II) was not significantly affected. The addition of TU or betaine anhydrous remarkably decreased the adsorption ability for Pd(II), and contrastingly slight changes in the abilities to adsorb Ru and Rh were also observed. These results show that AMP03 has a significant potential for separation of platinoid elements from HNO solution.
solution.