Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Nakaniwa, Tetsuko*; Fukata, Harumi*; Inoue, Tatsuya*; Goda, Masaki*; Nakai, Ryoko*; Kirii, Yasuyuki*; Adachi, Motoyasu; Tamada, Taro; Segawa, Shinichi*; Kuroki, Ryota; et al.
Biochemistry, 51(42), p.8410 - 8421, 2012/10
Times Cited Count:18 Percentile:42.99(Biochemistry & Molecular Biology)Protein kinase is a vital drug target for the treatment of a wide range of diseases. To investigate the effect of cysteine mutation on the function, stability and structure of kinase, free cysteines of c-Jun N-terminal kinase 1 (JNK1) were systematically removed by mutation. Two cysteine-destructed mutants in which three (M3) and seven (M7) cysteine residues are removed, yielded about 5 and 2 times than wild type JNK-1 (M0). SDS PAGE analysis showed that the aggregation was less in the case of M3 and M7. Thermal unfolding experiment of M0, M3 and M7 using by differential scanning calorimetry proceeded at least three state unfolding. Crystal structure of the M3 mutant was determined to 2.6  resolution, which was identical to that of the wild-type. Consequently, due to the highest yield, its improved stability against aggregation and its structural similarity to the wild type, the M3 mutant is suitable for the use of further characterization of its function and structure.
resolution, which was identical to that of the wild-type. Consequently, due to the highest yield, its improved stability against aggregation and its structural similarity to the wild type, the M3 mutant is suitable for the use of further characterization of its function and structure.
Tamada, Taro; Kinoshita, Takayoshi*; Tada, Toshiji*; Kuroki, Ryota
Nihon Kessho Gakkai-Shi, 52(2), p.133 - 138, 2010/04
To help resolve long-standing questions regarding the catalytic activity of the serine proteases the structure of porcine pancreatic elastase has been analyzed by high-resolution neutron (1.65  resolution) and X-ray (0.94
resolution) and X-ray (0.94  resolution) crystallography. In order to mimic the tetrahedral transition intermediate, a peptidic inhibitor was used. The neutron and X-ray data show that the hydrogen bond between His57 and Asp102 is not consistent with a low-barrier hydrogen which is predicted to have the hydrogen midway between the donor and acceptor atom. The neutron analysis also shows that the oxygen of the oxopropyl group of the inhibitor is present as an oxygen anion rather than a hydroxyl group, supporting the role of the "oxyanion hole" in stabilizing the tetrahedral intermediate in catalysis.
resolution) crystallography. In order to mimic the tetrahedral transition intermediate, a peptidic inhibitor was used. The neutron and X-ray data show that the hydrogen bond between His57 and Asp102 is not consistent with a low-barrier hydrogen which is predicted to have the hydrogen midway between the donor and acceptor atom. The neutron analysis also shows that the oxygen of the oxopropyl group of the inhibitor is present as an oxygen anion rather than a hydroxyl group, supporting the role of the "oxyanion hole" in stabilizing the tetrahedral intermediate in catalysis.
Tamada, Taro; Kinoshita, Takayoshi*; Kurihara, Kazuo; Adachi, Motoyasu; Ohara, Takashi; Imai, Keisuke*; Kuroki, Ryota; Tada, Toshiji*
Journal of the American Chemical Society, 131(31), p.11033 - 11040, 2009/07
Times Cited Count:58 Percentile:79.01(Chemistry, Multidisciplinary)To help resolve long-standing questions regarding the catalytic activity of the serine proteases the structure of porcine pancreatic elastase has been analyzed by high-resolution neutron and X-ray crystallography. In order to mimic the tetrahedral transition intermediate a peptidic inhibitor was used. A single large crystal was used to collect room-temperature neutron data to 1.65  resolution and X-ray data to 1.20
resolution and X-ray data to 1.20  resolution. Another crystal provided a low-temperature X-ray data set to 0.94
resolution. Another crystal provided a low-temperature X-ray data set to 0.94  resolution. The neutron data are to higher resolution than previously reported for a serine protease and the X-ray data are comparable with other studies. The neutron and X-ray data show that the hydrogen bond between His57 and Asp102 (chymotrypsin numbering) is 2.60
resolution. The neutron data are to higher resolution than previously reported for a serine protease and the X-ray data are comparable with other studies. The neutron and X-ray data show that the hydrogen bond between His57 and Asp102 (chymotrypsin numbering) is 2.60  in length and that the hydrogen-bonding hydrogen is 0.80-0.96
in length and that the hydrogen-bonding hydrogen is 0.80-0.96  from the histidine nitrogen. This is not consistent with a low-barrier hydrogen which is predicted to have the hydrogen midway between the donor and acceptor atom. The observed interaction between His57 and Asp102 is essentially a short but conventional hydrogen bond, sometimes described as a short ionic hydrogen bond. The neutron analysis also shows that the oxygen of the oxopropyl group of the inhibitor is present as an oxygen anion rather than a hydroxyl group, supporting the role of the "oxyanion hole" in stabilizing the tetrahedral intermediate in catalysis.
from the histidine nitrogen. This is not consistent with a low-barrier hydrogen which is predicted to have the hydrogen midway between the donor and acceptor atom. The observed interaction between His57 and Asp102 is essentially a short but conventional hydrogen bond, sometimes described as a short ionic hydrogen bond. The neutron analysis also shows that the oxygen of the oxopropyl group of the inhibitor is present as an oxygen anion rather than a hydroxyl group, supporting the role of the "oxyanion hole" in stabilizing the tetrahedral intermediate in catalysis.
 Ni
Ni Al
Al
Haga, Yoshinori; Matsuda, Tatsuma; Ikeda, Shugo; Galatanu, A.; Matsumoto, Takuya*; Sugimoto, Toyonari*; Tada, Toshiji*; Noguchi, Satoru*; Onuki, Yoshichika
Physica B; Condensed Matter, 359-361, p.1006 - 1008, 2005/04
Times Cited Count:2 Percentile:11.66(Physics, Condensed Matter)A new ternary uranium-based intermetallic compound U Ni
Ni Al
Al has been symthesized. It crystallizes in the unique flat orthorhombic structure. Uranium atoms occupy two crystallographic 4
has been symthesized. It crystallizes in the unique flat orthorhombic structure. Uranium atoms occupy two crystallographic 4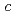 and 8
and 8 sites where local chemical environments are quite similar. The temperature dependence of the magnetic susceptibility
sites where local chemical environments are quite similar. The temperature dependence of the magnetic susceptibility 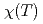 demonstrates peculiar magnetic anisotropy;
demonstrates peculiar magnetic anisotropy; 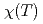 along
along  - and
- and 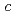 -axes obeys the Curie-Weiss law above 23 K, while
-axes obeys the Curie-Weiss law above 23 K, while 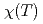 along
along 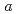 -axis is small and temperature independent. At 23 K, only
-axis is small and temperature independent. At 23 K, only 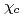 shows a sharp cusp corresponding to the antiferromagnetic ordering, while
shows a sharp cusp corresponding to the antiferromagnetic ordering, while 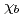 remains paramagnetic behavior down to 2 K. These results and crystallographical considerations lead to a conclusion that only uranium atoms at the 8
remains paramagnetic behavior down to 2 K. These results and crystallographical considerations lead to a conclusion that only uranium atoms at the 8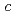 site order antiferromagnetically at
site order antiferromagnetically at 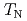 = 23 K, while uranium atoms at the
= 23 K, while uranium atoms at the 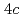 site do not order down to 50 mK.
site do not order down to 50 mK.
Tamada, Taro; Kinoshita, Takayoshi*; Kuroki, Ryota; Tada, Toshiji*
no journal, ,
no abstracts in English
Tamada, Taro; Kinoshita, Takayoshi*; Tada, Toshiji*; Kurihara, Kazuo; Ohara, Takashi; Kuroki, Ryota
no journal, ,
no abstracts in English
Tamada, Taro; Kinoshita, Takayoshi*; Ohara, Takashi; Kurihara, Kazuo; Tada, Toshiji*; Kuroki, Ryota
no journal, ,
no abstracts in English
Tamada, Taro; Kinoshita, Takayoshi*; Ohara, Takashi; Kurihara, Kazuo; Tada, Toshiji*; Kuroki, Ryota
no journal, ,
no abstracts in English
Tamada, Taro; Kinoshita, Takayoshi*; Ohara, Takashi; Kurihara, Kazuo; Imai, Keisuke*; Kuroki, Ryota; Tada, Toshiji*
no journal, ,
Porcine pancreatic elastase (PPE) is a serine protease classified in the chymotrypsin family. We determined two crystal structures of PPE in complex with peptidic inhibitor FR130180, which mimics the tetrahedral transition intermediate. One is the structure determined using 1.65  resolution neutron diffraction in the combination with 1.20
resolution neutron diffraction in the combination with 1.20  resolution X-ray diffraction data obtained using the same crystal. The other is the sub-angstrom X-ray structure determined to 0.94
resolution X-ray diffraction data obtained using the same crystal. The other is the sub-angstrom X-ray structure determined to 0.94  resolution. The structural features obtained from neutron diffraction and X-ray diffraction were compared to understand the detailed scheme of interaction between PPE and its inhibitor. From the observation of hydrogen atom located between the active site His57 and Asp102 by neutron and high resolution X-ray diffraction experiment, it is revealed that the interaction is not a low barrier hydrogen bond, but a short ionic hydrogen bond. Moreover, using neutron diffraction data we show that the hydroxyl group of inhibitor FR13080 bound within the "oxy-anion hole" exhibits an oxy-anion-like tetrahedral intermediate.
resolution. The structural features obtained from neutron diffraction and X-ray diffraction were compared to understand the detailed scheme of interaction between PPE and its inhibitor. From the observation of hydrogen atom located between the active site His57 and Asp102 by neutron and high resolution X-ray diffraction experiment, it is revealed that the interaction is not a low barrier hydrogen bond, but a short ionic hydrogen bond. Moreover, using neutron diffraction data we show that the hydroxyl group of inhibitor FR13080 bound within the "oxy-anion hole" exhibits an oxy-anion-like tetrahedral intermediate.
Tamada, Taro; Kinoshita, Takayoshi*; Kurihara, Kazuo; Adachi, Motoyasu; Ohara, Takashi; Tada, Toshiji*; Kuroki, Ryota
no journal, ,
To help resolve long-standing questions regarding the catalytic activity of the serine proteases the structure of porcine pancreatic elastase has been analyzed by high-resolution neutron and X-ray crystallography. In order to mimic the tetrahedral transition intermediate a peptidic inhibitor was used. A single large crystal was used to collect room-temperature neutron data to 1.65  resolution and X-ray data to 1.20
resolution and X-ray data to 1.20  resolution. Another crystal provided a low-temperature X-ray data set to 0.94
resolution. Another crystal provided a low-temperature X-ray data set to 0.94  resolution. The neutron data are to higher resolution than previously reported for a serine protease and the X-ray data are comparable with other studies. The neutron and X-ray data show that the hydrogen bond between His57 and Asp102 is 2.60
resolution. The neutron data are to higher resolution than previously reported for a serine protease and the X-ray data are comparable with other studies. The neutron and X-ray data show that the hydrogen bond between His57 and Asp102 is 2.60  in length and that the hydrogen-bonding hydrogen is 0.80-0.96
in length and that the hydrogen-bonding hydrogen is 0.80-0.96  from the histidine nitrogen. This is not consistent with a low-barrier hydrogen which is predicted to have the hydrogen midway between the donor and acceptor atom. The observed interaction between His57 and Asp102 is essentially a short but conventional hydrogen bond, sometimes described as a short ionic hydrogen bond. The neutron analysis also shows that the oxygen of the oxopropyl group of the inhibitor is present as an oxygen anion rather than a hydroxyl group, supporting the role of the "oxyanion hole" in stabilizing the tetrahedral intermediate in catalysis.
from the histidine nitrogen. This is not consistent with a low-barrier hydrogen which is predicted to have the hydrogen midway between the donor and acceptor atom. The observed interaction between His57 and Asp102 is essentially a short but conventional hydrogen bond, sometimes described as a short ionic hydrogen bond. The neutron analysis also shows that the oxygen of the oxopropyl group of the inhibitor is present as an oxygen anion rather than a hydroxyl group, supporting the role of the "oxyanion hole" in stabilizing the tetrahedral intermediate in catalysis.
Nakaniwa, Tetsuko*; Fukata, Harumi*; Inoue, Tatsuya*; Kinoshita, Takayoshi*; Adachi, Motoyasu; Tamada, Taro; Kuroki, Ryota; Tada, Toshiji*
no journal, ,
JNK1 is a MAP kinase responsible for response of stress. JNK1 has 4 and 3 cysteine residues in embedded region and at molecular surface, respectively. Those cysteine residues would cause inactivation and aggregation of the molecule. Based of the analysis of packing in crystal of isozyme of JNK1, we found more salt bridge and hydrogen bonding interactions on the interface. In this study, we focus on the two cysteine residues and introduced modification into M3 mutant previously reported.
Tamada, Taro; Kinoshita, Takayoshi*; Yamada, Mitsugu; Kurihara, Kazuo; Ohara, Takashi; Adachi, Motoyasu; Tada, Toshiji*; Kuroki, Ryota
no journal, ,
no abstracts in English
Tamada, Taro; Kinoshita, Takayoshi*; Kurihara, Kazuo; Tada, Toshiji*; Kuroki, Ryota
no journal, ,
Elastase is a serine protease classified in the chymotrypsin family, and is attractive target for studies of structure based drug design (SBDD). The structural information including hydrogen positions and hydration will help us to further elucidate the catalytic mechanism of serine protease. To obtain such structural information, we performed the neutron structure analyses of porcine pancreatic elastase (PPE) with and without its inhibitor using diffraction data obtained at a BIX-3 diffractometer in the research reactor JRR-3. The PPE structure in complex with/without peptidic inhibitor, which was used to mimic the tetrahedral intermediate state, was determined to 1.65/1.90  resolution, respectively. This structural information allows us to understand the role of resting state upon the catalytic reaction. Furthermore, the structural change of the active site residues including hydration structure obtained from the comparison between structures with and without inhibitor may help designing potent inhibitors by SBDD.
resolution, respectively. This structural information allows us to understand the role of resting state upon the catalytic reaction. Furthermore, the structural change of the active site residues including hydration structure obtained from the comparison between structures with and without inhibitor may help designing potent inhibitors by SBDD.