Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Takahashi, Tomoyuki*; Fukaya, Yukiko*; Iimoto, Takeshi*; Uni, Yasuo*; Kato, Tomoko; Sun, S.*; Takeda, Seiji; Nakai, Kunihiro*; Nakabayashi, Ryo*; Uchida, Shigeo*; et al.
Hoken Butsuri (Internet), 56(4), p.288 - 305, 2021/12
We report the results of activities related to the Task Group of Parameters Used in Biospheric Dose Assessment Models for Radioactive Waste Disposal at the Japan Health Physics Society.
Komori, Yukiko*; Yokokita, Takuya*; Kasamatsu, Yoshitaka*; Haba, Hiromitsu*; Toyoshima, Atsushi; Toyomura, Keigo*; Nakamura, Kohei*; Kanaya, Jumpei*; Huang, M.*; Kudo, Yuki*; et al.
Journal of Radioanalytical and Nuclear Chemistry, 303(2), p.1385 - 1388, 2015/02
Times Cited Count:2 Percentile:17.57(Chemistry, Analytical)We studied solid-liquid extraction behavior of carrier-free Mo and W radiotracers onto an Aliquat 336-loaded resin from HCl solutions toward extraction chromatography experiments of element 106, seaborgium(Sg). Distribution coefficients (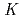
 ) of Mo and W on the resin were determined as a function of HCl concentration by a batch method. On-line extraction chromatography of Mo and W was also carried out in 2-8 M HCl solutions with an automated rapid chemistry apparatus. The order of extraction probability from 2 to 8 M HCl was Mo
) of Mo and W on the resin were determined as a function of HCl concentration by a batch method. On-line extraction chromatography of Mo and W was also carried out in 2-8 M HCl solutions with an automated rapid chemistry apparatus. The order of extraction probability from 2 to 8 M HCl was Mo W, which reflected the order of the
W, which reflected the order of the 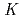
 values in the batch experiment.
values in the batch experiment.
Haraga, Tomoko; Saito, Shingo*; Sato, Yoshiyuki; Asai, Shiho; Hanzawa, Yukiko; Hoshino, Hitoshi*; Shibukawa, Masami*; Ishimori, Kenichiro; Takahashi, Kuniaki
Analytical Sciences, 30(7), p.773 - 776, 2014/07
Times Cited Count:6 Percentile:21.4(Chemistry, Analytical)A simple and rapid method with low radiation exposure risk was developed for the determination of neodymium in spent nuclear fuel by CE with LIF detection using a fluorescent ligand having a macrocyclic hexadentate polyaminocarboxylate structure. The concentration of Nd(III) in a spent nuclear fuel sample was determined with no interference from various matrix elements, including lanthanides and uranium (at a 200-fold excess), with 92  3% recovery. This is due to method's high resolution based on establishing a ternary complex equilibrium during migration in which the hydroxyl ion plays an auxiliary role.
3% recovery. This is due to method's high resolution based on establishing a ternary complex equilibrium during migration in which the hydroxyl ion plays an auxiliary role.
Kasai, Shinya*; Hirayama, Yusuke*; Takahashi, Yukiko*; Mitani, Seiji*; Hono, Kazuhiro*; Adachi, Hiroto; Ieda, Junichi; Maekawa, Sadamichi
Applied Physics Letters, 104(16), p.162410_1 - 162410_4, 2014/04
Times Cited Count:14 Percentile:52.23(Physics, Applied)Hayashi, Masamitsu*; Ieda, Junichi; Yamane, Yuta; Oe, Junichiro*; Takahashi, Yukiko*; Mitani, Seiji*; Maekawa, Sadamichi
Physical Review Letters, 108(14), p.147202_1 - 147202_5, 2012/04
Times Cited Count:37 Percentile:82.18(Physics, Multidisciplinary)Spinmotive force associated with a moving domain wall is observed directly in permalloy nanowires using real time voltage measurements with proper subtraction of the electromotive force. Whereas the wall velocity exhibits nonlinear dependence on a magnetic field, the generated voltage increases linearly with the field. We show that the sign of the voltage reverses when the wall propagation direction is altered. Numerical simulations explain quantitatively these features of spinmotive force and indicate that the spinmotive force scales with the field even in a field range where the wall motion is no longer associated with periodic structure transformation.
Konishi, Tomoya*; Nishiwaki, Nagatoshi*; Tojo, Takashi*; Ishikawa, Takuma*; Teraoka, Teruki*; Ueta, Yukiko*; Kihara, Yoshifumi*; Moritoki, Hideji*; Tono, Tatsuo*; Musashi, Mio*; et al.
Physica Status Solidi (C), 8(2), p.405 - 407, 2011/02
Times Cited Count:3 Percentile:74.8(Engineering, Electrical & Electronic)Hanzawa, Yukiko; Magara, Masaaki; Watanabe, Kazuo; Esaka, Fumitaka; Miyamoto, Yutaka; Yasuda, Kenichiro; Gunji, Katsubumi*; Yamamoto, Yoichi; Takahashi, Tsukasa; Sakurai, Satoshi; et al.
JAERI-Tech 2002-103, 141 Pages, 2003/02
The JAERI has established a facility with a cleanroom: the Clean Laboratory for Environmental Analysis and Research (CLEAR). This report is an overview of the design, construction and performance evaluation of the CLEAR in the initial stage of the laboratory operation in June 2001. The CLEAR is a facility to be used for ultra trace analyses of nuclear materials in environmental samples for the safeguards, for the CTBT verification and for researches on environmental sciences. The CLEAR meets double requirements of a cleanroom and for handling of nuclear materials. Much attention was paid to the construction materials of the cleanroom for trace analysis of metal elements using corrosive acids. The air conditioning and purification system, experimental equipment, utilities and safety systems are also demonstrated. The potential contamination from the completed cleanroom atmosphere during the analytical procedure was evaluated. It can be concluded that the CLEAR has provided a suitable condition for reliable analysis of ultra trace amounts of nuclear materials in environmental samples.
Magara, Masaaki; Sakakibara, Takaaki; Kurosawa, Setsumi; Takahashi, Masato; Sakurai, Satoshi; Hanzawa, Yukiko; Esaka, Fumitaka; Watanabe, Kazuo; Usuda, Shigekazu
Journal of Analytical Atomic Spectrometry, 17(9), p.1157 - 1160, 2002/09
Times Cited Count:11 Percentile:44.18(Chemistry, Analytical)no abstracts in English
Esaka, Fumitaka; Magara, Masaaki; Hanzawa, Yukiko; Sakurai, Satoshi; Taguchi, Takuji; Takai, Konomi; Sakakibara, Takaaki; Kurosawa, Setsumi; Takahashi, Masato; Yasuda, Kenichiro; et al.
Dai-22-Kai Kaku Busshitsu Kanri Gakkai Nihon Shibu Nenji Taikai Rombunshu, 8 Pages, 2001/11
no abstracts in English
Usuda, Shigekazu; Watanabe, Kazuo; Sakurai, Satoshi; Magara, Masaaki; Hanzawa, Yukiko; Esaka, Fumitaka; Miyamoto, Yutaka; Yasuda, Kenichiro; Saito, Yoko; Gunji, Katsubumi*; et al.
KEK Proceedings 2001-14, p.88 - 92, 2001/06
no abstracts in English
Adachi, Takeo; Usuda, Shigekazu; Watanabe, Kazuo; Sakurai, Satoshi; Magara, Masaaki; Hanzawa, Yukiko; Esaka, Fumitaka; Yasuda, Kenichiro; Saito, Yoko; Takahashi, Masato; et al.
IAEA-SM-367/10/02 (CD-ROM), 8 Pages, 2001/00
no abstracts in English
Nishimura, Hideo; Magara, Masaaki; Hanzawa, Yukiko; Esaka, Fumitaka; Takahashi, Tsukasa; Gunji, Katsubumi; Miyamoto, Yutaka; Yasuda, Kenichiro; Tsuruta, Yasuhiro; Tsuda, Shinji; et al.
Heisei 11-Nendo Hosho Sochi Semina Koenroku Tekisuto, p.95 - 107, 2000/01
no abstracts in English
Hanzawa, Yukiko; Magara, Masaaki; Esaka, Fumitaka; Watanabe, Kazuo; Usuda, Shigekazu; Gunji, Katsubumi; Yasuda, Kenichiro; Takahashi, Tsukasa; Nishimura, Hideo; Adachi, Takeo; et al.
Proceedings of the Institute of Nuclear Materials Management 40th Annual Meeting (CD-ROM), 7 Pages, 1999/00
no abstracts in English
Takayama, Reona*; Oe, Kazuhiro*; Komori, Yukiko*; Fujisawa, Hiroyuki*; Kuriyama, Ai*; Kikutani, Yuki*; Kikunaga, Hidetoshi*; Kasamatsu, Yoshitaka*; Yoshimura, Takashi*; Takahashi, Naruto*; et al.
no journal, ,
The extraction behavior of trivalent actinides (Am, Cm, Cf, Es, and Fm) into di(2-ethylhexyl)phosphoric acid, HDEHP, in benzene from HNO was studied together with lanthanides. The extraction constants,
was studied together with lanthanides. The extraction constants, 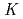
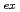 values, of lanthanides, Am, Cm, Cf, and Es were determined by a batch method using stable lanthanide isotopes and radiotracers of
values, of lanthanides, Am, Cm, Cf, and Es were determined by a batch method using stable lanthanide isotopes and radiotracers of  Am,
Am,  Cm,
Cm,  Cf, and
Cf, and 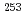 Es, respectively, while that of Fm was measured with
Es, respectively, while that of Fm was measured with 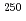 Fm which was produced in the
Fm which was produced in the  U(
U(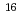 O,4n) reaction and was on-line supplied by a gas-jet method at the AVF cyclotron of RCNP, Osaka University. The
O,4n) reaction and was on-line supplied by a gas-jet method at the AVF cyclotron of RCNP, Osaka University. The 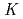
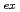 values were determined by the slope analysis for the variation of the distribution ratio as a function of the concentration of HDEHP. We found that the
values were determined by the slope analysis for the variation of the distribution ratio as a function of the concentration of HDEHP. We found that the 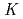
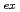 of Fm is smaller than that of Dy in spite of the similar ionic radii while those of other actinides are almost the same as those of each lanthanide with a similar ionic radius.
of Fm is smaller than that of Dy in spite of the similar ionic radii while those of other actinides are almost the same as those of each lanthanide with a similar ionic radius.
Yokokita, Takuya*; Komori, Yukiko*; Kikutani, Yuki*; Kino, Aiko*; Shiohara, Naoya*; Kasamatsu, Yoshitaka*; Yoshimura, Takashi*; Takahashi, Naruto*; Oe, Kazuhiro; Shinohara, Atsushi*
no journal, ,
We are planning to study a redox behavior of element 106, seaborgium (Sg) based on the comparison of the behaviors with those of molybdenum (Mo) and tungsten (W). In this work, we studied solvent extraction behaviors of Mo(VI), Mo(V), W(VI), and W(V) with Aliquat 336 from hydrochloric acid (HCl). Mo(V) is extracted with higher distribution ratios than those of Mo(VI) in 4 to 11 M HCl. Distribution ratios of Mo(V) obtained by reducing Mo(VI) electrochemically are in good agreement with those of [MoCl ].
].
Yokokita, Takuya*; Oe, Kazuhiro; Komori, Yukiko*; Kikutani, Yuki*; Kino, Aiko*; Nakamura, Kohei*; Kasamatsu, Yoshitaka*; Takahashi, Naruto*; Yoshimura, Takashi*; Takamiya, Koichi*; et al.
no journal, ,
We carried out solvent extraction of Mo(VI), Mo(V), W(VI) and W(V) in 0.01-0.36 M Aliquat 336 / 0.1-11 M HCl system as model experiments for element 106, seaborgium (Sg). The HCl solutions of Mo(VI), Mo(V), W(VI) and W(V) were prepared using macro amounts of Mo and W. Extraction behaviors of mononuclear Mo and W were also investigated using carrier-free radiotracers  Mo and
Mo and  W, which were produced as
W, which were produced as  U(n, f) and
U(n, f) and  Ta (p, n) reaction, respectively. The distribution ratios (D) of Mo(V) and W(V) were higher than those of Mo(VI) and W(VI), respectively. These results suggest that reduction behavior of the group-6 elements can be observed by solvent extraction. The D values of carrier-free Mo(VI) and W(VI) are almost the same as those with macro amounts in 6-11 M HCl. This condition would be suitable for the Sg experiments.
Ta (p, n) reaction, respectively. The distribution ratios (D) of Mo(V) and W(V) were higher than those of Mo(VI) and W(VI), respectively. These results suggest that reduction behavior of the group-6 elements can be observed by solvent extraction. The D values of carrier-free Mo(VI) and W(VI) are almost the same as those with macro amounts in 6-11 M HCl. This condition would be suitable for the Sg experiments.
 -ray emitters including
-ray emitters including  Cs, 1; Collaborative experiment by the committee on preparation of reference materials for radioactivity analysis of JSAC
Cs, 1; Collaborative experiment by the committee on preparation of reference materials for radioactivity analysis of JSACYonezawa, Chushiro*; Kakita, Kazutoshi*; Takahashi, Takanori*; Aono, Tatsuo*; Maeda, Satoshi; Abe, Takaaki*; Arakawa, Fumihiro*; Kiho, Nobuharu*; Akiyama, Masakazu*; Muramatsu, Isamu*; et al.
no journal, ,
no abstracts in English
Sugimoto, Satoshi*; Araki, Yasufumi; Takahashi, Yukiko*; Ieda, Junichi; Kasai, Shinya*
no journal, ,
no abstracts in English