Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
 O
O -pentadentate planar ligands designed for the strongest and selective capture of uranium from seawater
-pentadentate planar ligands designed for the strongest and selective capture of uranium from seawaterMizumachi, Takumi*; Sato, Minami*; Kaneko, Masashi; Takeyama, Tomoyuki*; Tsushima, Satoru*; Takao, Koichiro*
Inorganic Chemistry, 61(16), p.6175 - 6181, 2022/04
Times Cited Count:2 Percentile:36.89(Chemistry, Inorganic & Nuclear)Based on unique 5-fold equatorial coordination of UO
 , water-compatible pentadentate planar ligands, H
, water-compatible pentadentate planar ligands, H saldian and its derivatives, were designed as strong and selective capture of UO
saldian and its derivatives, were designed as strong and selective capture of UO
 in seawater. In the simulated seawater condition (0.5 M NaCl + 2.3 mM HCO
in seawater. In the simulated seawater condition (0.5 M NaCl + 2.3 mM HCO
 /CO
/CO
 , pH 8), saldian
, pH 8), saldian shows the strongest complexation with UO
shows the strongest complexation with UO
 to form UO
to form UO (saldian) (log
(saldian) (log
 = 28.05
= 28.05  0.07), which is more than 10 order of magnitude greater than amidoxime-based or -inspired ligand systems most commonly employed for U capture from seawater. Good selectivity for UO
0.07), which is more than 10 order of magnitude greater than amidoxime-based or -inspired ligand systems most commonly employed for U capture from seawater. Good selectivity for UO
 from other metal ions coexisting in seawater was also demonstrated.
from other metal ions coexisting in seawater was also demonstrated.
Ouchi, Kazuki; Komatsu, Atsushi; Takao, Koichiro*; Kitatsuji, Yoshihiro; Watanabe, Masayuki
Chemistry Letters, 50(6), p.1169 - 1172, 2021/06
Times Cited Count:1 Percentile:6.77(Chemistry, Multidisciplinary)The electrochemical behavior of uranium (IV) tetrachloride in ionic liquid-DMF mixture was studied for first time in order to build a redox flow battery (RFB) using U as an electrode active material. We found a quasi-reversible U /U
/U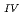 couple that could be applied to the anode reaction of the RFB.
couple that could be applied to the anode reaction of the RFB.
Suzuki, Tomoya; Takao, Koichiro*; Kawasaki, Takeshi*; Harada, Masayuki*; Nogami, Masanobu*; Ikeda, Yasuhisa*
Polyhedron, 96, p.102 - 106, 2015/08
Times Cited Count:5 Percentile:41.5(Chemistry, Inorganic & Nuclear)We have determined crystal structures of UO (NO
(NO )
) (
(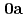 )
) (
(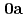 : 2-imidazolidone), UO
: 2-imidazolidone), UO (NO
(NO )
) (
(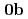 )
) (
(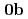 : tetrahydro-2-pyrimidone) and UO
: tetrahydro-2-pyrimidone) and UO (NO
(NO )
) (
(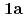 )
) (
(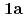 : 1-methyl-2-imidazolidone) by using single crystal X-ray analysis, and examined correlations between melting points (mps) and intermolecular hydrogen bonds (HBs) of UO
: 1-methyl-2-imidazolidone) by using single crystal X-ray analysis, and examined correlations between melting points (mps) and intermolecular hydrogen bonds (HBs) of UO (NO
(NO )
) (CU)
(CU) (CU: cyclic urea derivatives) and UO
(CU: cyclic urea derivatives) and UO (NO
(NO )
) (NRP)
(NRP) (NRP: pyrrolidone derivative).
(NRP: pyrrolidone derivative).
 -alkylated 2-pyrrolidone derivatives
-alkylated 2-pyrrolidone derivativesTakao, Koichiro*; Kawata, Yoshihisa*; Nogami, Masanobu*; Harada, Masayuki*; Morita, Yasuji; Nishimura, Kenji*; Ikeda, Yasuhisa*
Journal of Nuclear Science and Technology, 52(2), p.294 - 298, 2015/02
Times Cited Count:2 Percentile:17.57(Nuclear Science & Technology)Yields of precipitated UO (NO
(NO )
) (NRP)
(NRP) (NRP =
(NRP =  -alkylated 2-pyrrolidone) were precisely determined by considering reduction of the solution volume through the precipitation, which can be estimated from difference in acid concentrations of the liquid phases before and after the precipitation. The studied NRPs were
-alkylated 2-pyrrolidone) were precisely determined by considering reduction of the solution volume through the precipitation, which can be estimated from difference in acid concentrations of the liquid phases before and after the precipitation. The studied NRPs were  -
- -butyl (NBP) and
-butyl (NBP) and  -
- -propyl (NProP) derivatives. In both systems, the precipitation yields precisely determined were always higher than those simply calculated from the ratio of uranium concentrations before and after the precipitation. However, the differences between them are in the range of 0.6% - 2.6%. If such a difference is practically negligible, the volume reduction through the precipitation does not have to be taken into account for simplicity of the analytical manipulation.
-propyl (NProP) derivatives. In both systems, the precipitation yields precisely determined were always higher than those simply calculated from the ratio of uranium concentrations before and after the precipitation. However, the differences between them are in the range of 0.6% - 2.6%. If such a difference is practically negligible, the volume reduction through the precipitation does not have to be taken into account for simplicity of the analytical manipulation.
 ]) solution of [EMI]
]) solution of [EMI] [UO
[UO (NO
(NO )
) ]; First spectrophotometric evidence for existence of [UO
]; First spectrophotometric evidence for existence of [UO (NO
(NO )
) ]
]
Sasaki, Kotoe*; Suzuki, Tomoya*; Arai, Tsuyoshi*; Takao, Koichiro*; Suzuki, Shinichi; Yaita, Tsuyoshi; Ikeda, Yasuhisa*
Chemistry Letters, 43(5), p.670 - 672, 2014/05
Times Cited Count:7 Percentile:25.99(Chemistry, Multidisciplinary)no abstracts in English
 and Pu
and Pu
Kim, S.-Y.; Takao, Koichiro*; Haga, Yoshinori; Yamamoto, Etsuji; Kawata, Yoshihisa; Morita, Yasuji; Nishimura, Kenji*; Ikeda, Yasuhisa*
Crystal Growth & Design, 10(5), p.2033 - 2036, 2010/04
Times Cited Count:15 Percentile:77.61(Chemistry, Multidisciplinary)Plutonyl(VI) nitrate complexes with N-cyclohexyl-2-pyrrolidone (NCP) and N-neopentyl-2-pyrrolidone (NNpP) were prepared, and their molecular and crystal structures were determined by single crystal X-ray analysis. The obtained compounds have the similar composition, PuO (NO
(NO )
) (NRP)
(NRP) (NRP = NCP, NNpP), which are analogous to the corresponding U(VI) complexes. Both PuO
(NRP = NCP, NNpP), which are analogous to the corresponding U(VI) complexes. Both PuO (NO
(NO )
) (NRP)
(NRP) complexes show typical structural properties of actinyl(VI) nitrates, i.e., hexagonal-bipyramidal geometry consisting of two NRP molecules and two NO
complexes show typical structural properties of actinyl(VI) nitrates, i.e., hexagonal-bipyramidal geometry consisting of two NRP molecules and two NO
 ions located in trans positions in the equatorial plane of PuO
ions located in trans positions in the equatorial plane of PuO
 moiety. These findings provide one of criteria in selection of suitable NRP as a precipitation agent for the spent nuclear fuel reprocessing based on the precipitation method.
moiety. These findings provide one of criteria in selection of suitable NRP as a precipitation agent for the spent nuclear fuel reprocessing based on the precipitation method.
Ikeda, Atsushi; Tsushima, Satoru*; Takao, Koichiro*; Rossberg, A.*; Funke, H.*; Scheinost, A. C.*; Bernhard, G.*; Yaita, Tsuyoshi; Hennig, C.*
Inorganic Chemistry, 48(24), p.11779 - 11787, 2009/12
Times Cited Count:34 Percentile:79.72(Chemistry, Inorganic & Nuclear)The electrochemical behavior and complex structure of Np carbonato complexes have been investigated in aqueous Na CO
CO and Na
and Na CO
CO /NaOH solutions at different oxidation states by using cyclic voltammetry, X-ray absorption spectroscopy, and density functional theory calculations. The end-member complexes of penta- and hexavalent Np in 1.5 M Na
/NaOH solutions at different oxidation states by using cyclic voltammetry, X-ray absorption spectroscopy, and density functional theory calculations. The end-member complexes of penta- and hexavalent Np in 1.5 M Na CO
CO with pH = 11.7 have been determined as a transdioxo neptunyl tricarbonato complex, [NpO
with pH = 11.7 have been determined as a transdioxo neptunyl tricarbonato complex, [NpO (CO
(CO )
) ]
]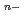 (
( = 5 for Np
= 5 for Np , and 4 for Np
, and 4 for Np ). In contrast, the electrochemical oxidation of Np
). In contrast, the electrochemical oxidation of Np in a highly basic carbonate solution of 2.0M Na
in a highly basic carbonate solution of 2.0M Na CO
CO /1.0M NaOH (pH
/1.0M NaOH (pH  13) yielded a stable heptavalent Np complex of [Np
13) yielded a stable heptavalent Np complex of [Np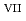 O
O (OH)
(OH) ]
] , indicating that the oxidation reaction from Np
, indicating that the oxidation reaction from Np to Np
to Np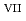 in the carbonate solution involves a drastic structural rearrangement from the transdioxo configuration to a square-planar-tetraoxo configuration, as well as exchanging the coordinating anions from carbonate ions (CO
in the carbonate solution involves a drastic structural rearrangement from the transdioxo configuration to a square-planar-tetraoxo configuration, as well as exchanging the coordinating anions from carbonate ions (CO
 ) to hydroxide ions (OH
) to hydroxide ions (OH ).
).
Morita, Yasuji; Takao, Koichiro*; Kim, S.-Y.; Kawata, Yoshihisa; Harada, Masayuki*; Nogami, Masanobu*; Nishimura, Kenji*; Ikeda, Yasuhisa*
Journal of Nuclear Science and Technology, 46(12), p.1129 - 1136, 2009/12
Times Cited Count:18 Percentile:74.76(Nuclear Science & Technology)A reprocessing system for spent FBR fuels consisting of two precipitation processes has been proposed. In this system, first only U(IV) species are precipitated using pyrrolidone derivative with low hydrophobicity and donicity, and secondly residual U(VI) and Pu(IV, VI) are precipitated simultaneously using pyrrolidone derivative with high precipitation ability. In this study, we have examined precipitation behavior of U(VI), Pu(IV), and Pu(VI) species in nitric acid solutions by using  -
- -propyl-2-pyrrolidone (NProP),
-propyl-2-pyrrolidone (NProP),  -butyl-2-pyrrolidone (NBP),
-butyl-2-pyrrolidone (NBP),  -
- -butyl-2-pyrrolidone (NiBP), or
-butyl-2-pyrrolidone (NiBP), or  -cyclohexyl-2-pyrrolidone (NCP) to select the precipitants for the first precipitation process. As a result, NBP were found to be the most promising precipitant for the first precipitation process.
-cyclohexyl-2-pyrrolidone (NCP) to select the precipitants for the first precipitation process. As a result, NBP were found to be the most promising precipitant for the first precipitation process.
 -Alkyl-2-pyrrolidone derivatives (Alkyl =
-Alkyl-2-pyrrolidone derivatives (Alkyl =  -propyl,
-propyl,  -butyl,
-butyl,  -butyl, and cyclohexyl)
-butyl, and cyclohexyl)Takao, Koichiro*; Noda, Kyoko*; Nogami, Masanobu*; Sugiyama, Yuichi*; Harada, Masayuki*; Morita, Yasuji; Nishimura, Kenji*; Ikeda, Yasuhisa*
Journal of Nuclear Science and Technology, 46(10), p.995 - 999, 2009/10
Times Cited Count:14 Percentile:66.94(Nuclear Science & Technology)We have been developing an advanced reprocessing system for spent FBR fuels based on precipitation method using pyrrolidone derivatives. In the present study, the solubility of UO (NO
(NO )
) (NRP)
(NRP) (NRP =
(NRP =  -alkyl-2-pyrrolidone, alkyl =
-alkyl-2-pyrrolidone, alkyl =  -propyl,
-propyl,  -butyl,
-butyl, 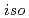 -butyl and cyclohexyl) in aqueous solutions with HNO
-butyl and cyclohexyl) in aqueous solutions with HNO has been examined. As a result, the solubility of each species of UO
has been examined. As a result, the solubility of each species of UO (NO
(NO )
) (NRP)
(NRP) generally decreases with increasing concentrations of HNO
generally decreases with increasing concentrations of HNO and NRP (
and NRP (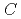 (HNO
(HNO ) and
) and 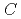 (NRP), respectively). The solubility of UO
(NRP), respectively). The solubility of UO (NO
(NO )
) (NRP)
(NRP) also depends on the type of NRP; a higher hydrophobicity of NRP generally leads to a lower solubility of UO
also depends on the type of NRP; a higher hydrophobicity of NRP generally leads to a lower solubility of UO (NO
(NO )
) (NRP)
(NRP) . The logarithms of effective solubility products (
. The logarithms of effective solubility products (
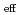 ) of UO
) of UO (NO
(NO )
) (NRP)
(NRP) at different
at different 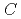 (HNO
(HNO ) values and 293 K were evaluated.
) values and 293 K were evaluated.
 -alkylated 2-pyrrolidone derivatives; Design and optimization of promising precipitant for uranyl ion
-alkylated 2-pyrrolidone derivatives; Design and optimization of promising precipitant for uranyl ionTakao, Koichiro*; Noda, Kyoko*; Morita, Yasuji; Nishimura, Kenji*; Ikeda, Yasuhisa*
Crystal Growth & Design, 8(7), p.2364 - 2376, 2008/07
Times Cited Count:35 Percentile:89.88(Chemistry, Multidisciplinary)Molecular and crystal structures of UO (NO
(NO )
) (NRP)
(NRP) (NRP = N-alkylated 2-pyrrolidone derivative) have been investigated by using single crystal X-ray analysis, IR and Raman spectroscopies. All UO
(NRP = N-alkylated 2-pyrrolidone derivative) have been investigated by using single crystal X-ray analysis, IR and Raman spectroscopies. All UO (NO
(NO )
) (NRP)
(NRP) complexes have typical structural properties of UO
complexes have typical structural properties of UO (NO
(NO )
) (L)
(L) (L = unidentate ligand), i.e., hexagonal-bipyramidal geometry, two NRP and two NO
(L = unidentate ligand), i.e., hexagonal-bipyramidal geometry, two NRP and two NO
 located in trans positions in an equatorial plane of the uranyl ion. Observation of the crystals of the uranyl nitrate complexes with N-n-propyl-2-pyrrolidone and N-iso-propyl-2-pyrrolidone indicate the presence of significant voids in the crystal lattices of these compounds. From this result, an approach for construction of efficient packing of UO
located in trans positions in an equatorial plane of the uranyl ion. Observation of the crystals of the uranyl nitrate complexes with N-n-propyl-2-pyrrolidone and N-iso-propyl-2-pyrrolidone indicate the presence of significant voids in the crystal lattices of these compounds. From this result, an approach for construction of efficient packing of UO (NO
(NO )
) (NRP)
(NRP) was proposed. Consequently, it was found that N-iso-butyl-2-pyrrolidone completely satisfies with the requirement for the efficient packing by filling the voids with the alkyl chain.
was proposed. Consequently, it was found that N-iso-butyl-2-pyrrolidone completely satisfies with the requirement for the efficient packing by filling the voids with the alkyl chain.
Ikeda, Yasuhisa*; Takao, Koichiro*; Harada, Masayuki*; Morita, Yasuji; Nogami, Masanobu*; Nishimura, Kenji*
Proceedings of International Conference on Advanced Nuclear Fuel Cycles and Systems (Global 2007) (CD-ROM), p.1503 - 1507, 2007/09
We have developed a reprocessing process for spent FBR fuels based on the precipitation method using pyrrolidone derivatives. In previous investigation, N-cyclohexyl-2-pyrrolidone (NCP) is used as a precipitant and a process consisting of selective U precipitation step and U-Pu co-precipitation step was developed. In the present study, in order to examine the applicability of precipitants with lower hydrophobicity than NCP to the selective U precipitation step, we have carried out precipitation experiments of U(VI) by N-butyl-2-pyrrolidone (NBP) and N-propyl-2-pyrrolidone (NProP) and measured decontamination factors of some fission products.
Noda, Kyoko*; Takao, Koichiro*; Sugiyama, Yuichi*; Harada, Masayuki*; Nogami, Masanobu*; Maruyama, Koichi*; Takahashi, Hiroaki*; Kim, S.-Y.; Sato, Makoto; Mineo, Hideaki; et al.
no journal, ,
We have been developing an advanced reprocessing system for spent FBR fuels based on precipitation method using pyrrolidone derivatives. In previous investigation, N-cyclohexyl-2-pyrrolidone (NCP) is used as a precipitant, which is able to precipitate selectively UO
 ions in HNO
ions in HNO solution, and a process consisting of two separation steps; selective U precipitation step and U-Pu co-precipitation step, was developed. In order to make the process more effective and more economical, we are now studying precipitation of U and Pu with other pyrrolidone derivatives. The outline of the study and main results obtained until now are shown in this presentation.
solution, and a process consisting of two separation steps; selective U precipitation step and U-Pu co-precipitation step, was developed. In order to make the process more effective and more economical, we are now studying precipitation of U and Pu with other pyrrolidone derivatives. The outline of the study and main results obtained until now are shown in this presentation.

 -precipitant from a viewpoint of crystallography
-precipitant from a viewpoint of crystallographyTakao, Koichiro*; Noda, Kyoko*; Nogami, Masanobu*; Sugiyama, Yuichi*; Harada, Masayuki*; Ikeda, Yasuhisa*; Morita, Yasuji; Nishimura, Kenji*
no journal, ,
We have been developing an advanced reprocessing system for spent FBR fuels based on precipitation method using pyrrolidone derivatives. Structural analyses of UO (NO
(NO )
) L
L (L=11 kinds of pyrrolidone derivatives) have been carried out using X-ray diffraction method to obtain some useful data for the selection of the optimum precipitant. It was found that the U(VI) complex with N-iso-butyl-2-pyrrolidone makes the most compact crystal.
(L=11 kinds of pyrrolidone derivatives) have been carried out using X-ray diffraction method to obtain some useful data for the selection of the optimum precipitant. It was found that the U(VI) complex with N-iso-butyl-2-pyrrolidone makes the most compact crystal.
Morita, Yasuji; Kim, S.-Y.; Kawata, Yoshihisa; Sato, Makoto; Ikeda, Yasuhisa*; Takao, Koichiro*; Noda, Kyoko*; Nishimura, Kenji*
no journal, ,
Precipitation behavior of Pu with pyrrolidone derivatives of N-(1,2-dimethyl)propyl-2-pyrrolidone (NDMProP) and N-neopenthyl-2-pyrrolidone (NNpP) in the solutions of U-Pu mixture has been examined in order to evaluate their applicability to the U-Pu co-precipitation process in the reprocessing based only on precipitation method. We have previously developed a process with N-cyclohexyl-2-pyrrolidone (NCP). It was found that U(VI) was precipitated in a high yield with any of the three precipitants and the precipitation yield of Pu(IV) was increased with the added amount of the precipitants. When NNpP was added with the ratio of [NNpP]/[U+Pu]=2.5, 99.5% of Pu was precipitate. Since NNpP showed the highest precipitation ability for Pu(IV) and the best physical property as precipitate, NNpP would be the most appropriate precipitant for the U-Pu co-precipitation process.
Takao, Koichiro*; Noda, Kyoko*; Nogami, Masanobu*; Sugiyama, Yuichi*; Harada, Masayuki*; Ikeda, Yasuhisa*; Morita, Yasuji; Nishimura, Kenji*
no journal, ,
Precipitation behavior of U(VI) and some fission products (FP) with pyrrolidone derivatives of N-(1,2-dimethyl)propyl-2-pyrrolidone (NDMProP) and N-neopenthyl-2-pyrrolidone (NNpP) in the solutions of U-FP mixture has been examined in order to evaluate their applicability to the U-Pu co-precipitation process in the reprocessing based only on precipitation method. We have previously developed a process with N-cyclohexyl-2-pyrrolidone (NCP). It was found that U(VI) was precipitated in a high yield with any of the three precipitants and the order of the precipitation ability for U(VI) was NCP NNpP
NNpP NDMProP. Decontamination factors (DF) against FP except Zr(IV) and Mo(VI) in the U(VI) precipitation were over 100, and therefore NNpP and NDMProP can be used as an alternative precipitants to NCP.
NDMProP. Decontamination factors (DF) against FP except Zr(IV) and Mo(VI) in the U(VI) precipitation were over 100, and therefore NNpP and NDMProP can be used as an alternative precipitants to NCP.
Nogami, Masanobu*; Noda, Kyoko*; Takao, Koichiro*; Sugiyama, Yuichi*; Harada, Masayuki*; Ikeda, Yasuhisa*; Kawata, Yoshihisa; Morita, Yasuji; Nishimura, Kenji*
no journal, ,
We have been developing an advanced reprocessing system for spent FBR fuels based on precipitation method using pyrrolidone derivatives. The present study deals with durability of new precipitants with low and high hydrophobicity against  -irradiation and heating. Results showed that the precipitants with low hydrophobicity, N-n-butyl-2-pyrrolidone, N-propyl-2-pyrrolidone and N-iso-butyl-2-pyrrolidone, have enough durability against
-irradiation and heating. Results showed that the precipitants with low hydrophobicity, N-n-butyl-2-pyrrolidone, N-propyl-2-pyrrolidone and N-iso-butyl-2-pyrrolidone, have enough durability against  -irradiation. The precipitants with high hydrophobicity, N-cyclohexyl-2-pyrrolidone, N-(1,2-dimethyl)propyl-2-pyrrolidone and N-neopenthyl-2-pyrrolidone, also have enough durability but gave lower precipitation yield when they irradiated with higher dose rate. The precipitation ability of all the precipitants did not changed by the heating at 50
-irradiation. The precipitants with high hydrophobicity, N-cyclohexyl-2-pyrrolidone, N-(1,2-dimethyl)propyl-2-pyrrolidone and N-neopenthyl-2-pyrrolidone, also have enough durability but gave lower precipitation yield when they irradiated with higher dose rate. The precipitation ability of all the precipitants did not changed by the heating at 50 C for three days.
C for three days.
Nogami, Masanobu*; Takao, Koichiro*; Sugiyama, Yuichi*; Noda, Kyoko*; Harada, Masayuki*; Ikeda, Yasuhisa*; Morita, Yasuji; Nishimura, Kenji*
no journal, ,
We have been developing an advanced reprocessing system for spent FBR fuels based on precipitation method using pyrrolidone derivatives. The present study deals with the effect of addition of masking agents on co-precipitation of Pu(IV) in the selective U(VI) precipitation. It was found that precipitation of U(IV), which was used as substitute for Pu(IV), was suppressed by the presence of acetohydoxamic acid.
Nogami, Masanobu*; Kawasaki, Takeshi*; Takao, Koichiro*; Noda, Kyoko*; Sugiyama, Yuichi*; Harada, Masayuki*; Ikeda, Yasuhisa*; Morita, Yasuji; Chikazawa, Takahiro*; Kikuchi, Toshiaki*; et al.
no journal, ,
A reprocessing system for spent FBR fuels based on the two precipitation processes has been proposed. In this system, first only U(VI) species are precipitated using pyrrolidone derivatives (NRP) with low hydrophobicity and donicity, and secondly residual U(VI) and Pu(IV, VI) are precipitated simultaneously using pyrrolidone derivative with high precipitation ability. In order to develop such a reprocessing method, behavior of uranyl ions in HNO has been studied using various novel NRP. In this presentation, recent advances on precipitation behavior and of uranyl ions and calcination of U(VI)-NRP will be introduced.
has been studied using various novel NRP. In this presentation, recent advances on precipitation behavior and of uranyl ions and calcination of U(VI)-NRP will be introduced.
Suzuki, Tomoya; Takao, Koichiro*; Kawasaki, Takeshi*; Sasaki, Yuji; Harada, Masayuki*; Nogami, Masanobu*; Ikeda, Yasuhisa*
no journal, ,
An advanced reprocessing system for spent FBR fuels has been proposed on the basis of selective precipitation ability of pyrrolidone or cyclic urea derivatives. In this process, U and Pu are recovered through two precipitation process after dissolution of spent FBR fuels in HNO solution. In our previous study, it was found that hydrophilic compounds which can form uranyl nitrate complexes with high melting points have high selective precipitation ability to U(VI). In this study, molecular and crystal structures of uranyl nitrate complexes bearing cyclic urea ligands were examined to clarify requirements for forming the U(VI) complexes with high melting points.
solution. In our previous study, it was found that hydrophilic compounds which can form uranyl nitrate complexes with high melting points have high selective precipitation ability to U(VI). In this study, molecular and crystal structures of uranyl nitrate complexes bearing cyclic urea ligands were examined to clarify requirements for forming the U(VI) complexes with high melting points.
Saeki, Morihisa; Matsumura, Daiju; Yomogida, Takumi; Taguchi, Tomitsugu; Tsuji, Takuya; Kusano, Shogo*; Miyazaki, Tatsuya*; Takao, Koichiro*; Oba, Hironori; Nakashima, Nobuaki*
no journal, ,
Recently, we found that photo-induced particle formation of Pd was promoted by coexistence of molybdenum anion in aqueous solution. In this study, we performed in-situ time-resolved XAFS measurement in the photoreduction of Pd
was promoted by coexistence of molybdenum anion in aqueous solution. In this study, we performed in-situ time-resolved XAFS measurement in the photoreduction of Pd in presence of various concentration of molybdenum anion. The effect of molybdenum addition on photo-induced particle formation of Pd will be discussed based on the relationship between the photoreduction rate of Pd
in presence of various concentration of molybdenum anion. The effect of molybdenum addition on photo-induced particle formation of Pd will be discussed based on the relationship between the photoreduction rate of Pd and the concentration of molybdenum anion.
and the concentration of molybdenum anion.